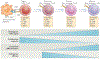Adoptive immunotherapy for cancer: harnessing the T cell response - PubMed (original) (raw)
Review
Adoptive immunotherapy for cancer: harnessing the T cell response
Nicholas P Restifo et al. Nat Rev Immunol. 2012.
Abstract
Immunotherapy based on the adoptive transfer of naturally occurring or gene-engineered T cells can mediate tumour regression in patients with metastatic cancer. Here, we discuss progress in the use of adoptively transferred T cells, focusing on how they can mediate tumour cell eradication. Recent advances include more accurate targeting of antigens expressed by tumours and the associated vasculature, and the successful use of gene engineering to re-target T cells before their transfer into the patient. We also describe how new research has helped to identify the particular T cell subsets that can most effectively promote tumour eradication.
Conflict of interest statement
Competing interests statement
The authors declare no competing financial interests.
Figures
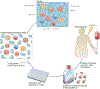
Figure 1 |. Isolation of tumour-infiltrating lymphocytes and expansion of tumour-specific T cell populations.
Tumours are often complex masses containing diverse cell types. These masses can be surgically resected and fragmented, and the cells can be placed in wells into which a T cell growth factor, such as interleukin-2 (IL-2), is added. T cell populations that have the desired T cell receptor (TCR) specificity can be selected and expanded, and then adoptively transferred into patients with cancer. Prior to this adoptive transfer, hosts can be immunodepleted by either chemotherapy alone or chemotherapy in combination with total-body irradiation. The combination of a lymphodepleting preparative regimen, adoptive cell transfer and a T cell growth factor (such as IL-2) can lead to prolonged tumour eradication in patients with metastatic melanoma. MDSC, myeloid-derived suppressor cell; NK, natural killer; TReg, regulatory T.
Figure 2 |. Three ways to genetically engineer T cells to confer specificity for tumour-associated antigens.
T cells can be genetically engineered to recognize tumour-associated antigens in various ways in current clinical trials. If a patient expresses a tumour-associated antigen that is recognized by an available receptor structure, autologous T cells can be genetically engineered to express the desired receptor. New receptors can be generated in a variety of ways. a | T cells can be identified and cloned from patients with particularly good antitumour responses. Their T cell receptors (TCRs) can be cloned and inserted into retroviruses or lentiviruses, which are then used to infect autologous T cells from the patient to be treated. b | Chimeric antigen receptors (CARs) can be generated in a variety of ways. Most commonly, sequences encoding the variable regions of antibodies are engineered to encode a single chain, which is then genetically engrafted onto the TCR intracellular domains that are capable of activating T cells. These CARs have antibody-like specificities, which enable them to recognize MHC-nonrestricted structures on the surfaces of target cells. c | TCRs can also be isolated from humanized mice that have been primed to recognize tumour antigens. These mice express human MHC class I or MHC class II molecules and can be immunized with the tumour antigen of interest. Mouse T cells specific for the MHC-restricted epitope of interest can then be isolated, and their TCR genes are cloned into recombinant vectors that can be used to genetically engineer autologous T cells from the patient.
Figure 3 |. Progressive T cell differentiation diminishes proliferative and antitumour capacities.
T cells experience progressive changes in their phenotypes following antigenic stimulation. Depending on the strength and duration of the signals that they encounter during activation, they are launched on a pathway of proliferation and differentiation. T cells must be able to fully differentiate if they are to have antitumour efficacy. However, experimental evidence indicates that for adoptive transfer, T cell differentiation is inversely correlated with antitumour efficacy. The process of T cell differentiation results in the loss of proliferative and self-renewal capacity. For CD8+ T cells, T memory stem (TSCM) cells are more effective against tumours than central memory T (TCM) cells, which are more effective than effector memory T (TEM) cells. APC, antigen-presenting cell; CCR7, CC-chemokine receptor 7; IL-2Rβ, IL-2 receptor β-chain; TCR, T cell receptor. Figure is modified, with permission, from REF. © (2011) Macmillan Publishers Ltd. All rights reserved.
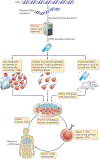
Figure 4 |. Highly personalized medicine.
Inexpensive and readily available DNA sequencing technology might revolutionize cancer immunotherapy, enabling a highly personalized approach to the identification of new tumour-associated antigens. The expressed genes from a patient’s tumour can be sequenced to identify candidate mutant T cell epitopes. Relevant epitopes that could potentially bind to the MHC molecules of the patient could be predicted using peptide prediction algorithms (for example, see the
HLA Peptide Binding Predictions website
). If peptides derived from mutant proteins are found to be capable of forming new MHC-restricted target structures, the candidate peptides could be used in one of at least three ways. First, scientists can identify or sort cells that express relevant antigens (such as those derived from driver oncogenes) using tetramer-like reagents. Second, candidate peptides could be used to stimulate T cells that are already present in the patient’s tumour or in their peripheral blood. Third, tumour antigens could be used to prime tumour-specific T cells in humanized mice that are transgenic for human MHC molecules. If the T cell populations generated are specific for the patient’s tumour, they could be expanded and adoptively transferred if they are of human origin. Alternatively, mouse T cells can be used to identify suitable T cell receptors (TCRs) for gene-engineering approaches. TIL, tumour-infiltrating lymphocyte.
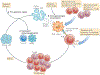
Figure 5 |. The rationale for combining targeted therapies with adoptive cell transfer-based immunotherapy.
a | A targeted agent (such as vemurafenib) can be used to promote apoptosis in tumour cells. b | Antigens released by dying tumour cells can then be acquired at an increased rate by antigen-presenting cells (APCs) that are present in the tissue or in local draining lymph nodes. These APCs process the tumour antigens and present tumour-derived peptides to T cells. This can lead to the priming of adoptively transferred tumour-specific T cells, as well as the activation of other endogenous tumour-specific T cell populations. Treatment with immunostimulatory cytokines and chemokines may increase the efficiency of tumour-specific T cell activation. c | Therapies that target immunosuppressive factors or cells present in the tumour microenvironment — such as regulatory T (TReg) cells and myeloid-derived suppressor cells (MDSCs) — may also promote increased activation of tumour-specific T cells. TCR, T cell receptor.
Similar articles
- Treating cancer with genetically engineered T cells.
Park TS, Rosenberg SA, Morgan RA. Park TS, et al. Trends Biotechnol. 2011 Nov;29(11):550-7. doi: 10.1016/j.tibtech.2011.04.009. Epub 2011 Jun 12. Trends Biotechnol. 2011. PMID: 21663987 Free PMC article. Review. - T-cell adoptive immunotherapy using tumor-infiltrating T cells and genetically engineered TCR-T cells.
Ikeda H. Ikeda H. Int Immunol. 2016 Jul;28(7):349-53. doi: 10.1093/intimm/dxw022. Epub 2016 Apr 28. Int Immunol. 2016. PMID: 27127191 Review. - Adoptive cell therapy: genetic modification to redirect effector cell specificity.
Morgan RA, Dudley ME, Rosenberg SA. Morgan RA, et al. Cancer J. 2010 Jul-Aug;16(4):336-41. doi: 10.1097/PPO.0b013e3181eb3879. Cancer J. 2010. PMID: 20693844 Free PMC article. Review. - Adoptive T cell transfer for cancer immunotherapy in the era of synthetic biology.
Kalos M, June CH. Kalos M, et al. Immunity. 2013 Jul 25;39(1):49-60. doi: 10.1016/j.immuni.2013.07.002. Immunity. 2013. PMID: 23890063 Free PMC article. Review. - Genetic redirection of T cells for cancer therapy.
Westwood JA, Kershaw MH. Westwood JA, et al. J Leukoc Biol. 2010 May;87(5):791-803. doi: 10.1189/jlb.1209824. Epub 2010 Feb 23. J Leukoc Biol. 2010. PMID: 20179152 Review.
Cited by
- Could the Adoptive Transfer of Memory Lymphocytes be an Alternative Treatment for Acinetobacter baumannii Infections?
Cebrero-Cangueiro T, Herrera-Espejo S, Paniagua M, Labrador-Herrera G, Cisneros JM, Pachón J, Pachón-Ibáñez ME. Cebrero-Cangueiro T, et al. Int J Mol Sci. 2024 Sep 30;25(19):10550. doi: 10.3390/ijms251910550. Int J Mol Sci. 2024. PMID: 39408879 Free PMC article. - Unlocking Apoptotic Pathways: Overcoming Tumor Resistance in CAR-T-Cell Therapy.
Zhang Z, Su M, Jiang P, Wang X, Tong X, Wu G. Zhang Z, et al. Cancer Med. 2024 Oct;13(19):e70283. doi: 10.1002/cam4.70283. Cancer Med. 2024. PMID: 39377542 Free PMC article. Review. - Unlocking the Potential: Epstein-Barr Virus (EBV) in Gastric Cancer and Future Treatment Prospects, a Literature Review.
Corallo S, Lasagna A, Filippi B, Alaimo D, Tortorella A, Serra F, Vanoli A, Pedrazzoli P. Corallo S, et al. Pathogens. 2024 Aug 28;13(9):728. doi: 10.3390/pathogens13090728. Pathogens. 2024. PMID: 39338919 Free PMC article. Review. - Immune-modulative nano-gel-nano system for patient-favorable cancer therapy.
Kim SH, Han RT, Han HS, Kim YM. Kim SH, et al. Bioact Mater. 2024 Sep 17;43:67-81. doi: 10.1016/j.bioactmat.2024.08.047. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39328776 Free PMC article. - Deficiency of metabolic regulator PKM2 activates the pentose phosphate pathway and generates TCF1+ progenitor CD8+ T cells to improve immunotherapy.
Markowitz GJ, Ban Y, Tavarez DA, Yoffe L, Podaza E, He Y, Martin MT, Crowley MJP, Sandoval TA, Gao D, Martin ML, Elemento O, Cubillos-Ruiz JR, McGraw TE, Altorki NK, Mittal V. Markowitz GJ, et al. Nat Immunol. 2024 Oct;25(10):1884-1899. doi: 10.1038/s41590-024-01963-1. Epub 2024 Sep 26. Nat Immunol. 2024. PMID: 39327500
References
- Deguine J, Breart B, Lemaitre F, Di Santo JP & Bousso P Intravital imaging reveals distinct dynamics for natural killer and CD8+ T cells during tumor regression. Immunity 33, 632–644 (2010). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources