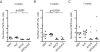Activated ALK collaborates with MYCN in neuroblastoma pathogenesis - PubMed (original) (raw)
. 2012 Mar 20;21(3):362-73.
doi: 10.1016/j.ccr.2012.02.010.
Jeong-Soo Lee, Feng Guo, Jimann Shin, Antonio R Perez-Atayde, Jeffery L Kutok, Scott J Rodig, Donna S Neuberg, Daniel Helman, Hui Feng, Rodney A Stewart, Wenchao Wang, Rani E George, John P Kanki, A Thomas Look
Affiliations
- PMID: 22439933
- PMCID: PMC3315700
- DOI: 10.1016/j.ccr.2012.02.010
Activated ALK collaborates with MYCN in neuroblastoma pathogenesis
Shizhen Zhu et al. Cancer Cell. 2012.
Abstract
Amplification of the MYCN oncogene in childhood neuroblastoma is often accompanied by mutational activation of ALK (anaplastic lymphoma kinase), suggesting their pathogenic cooperation. We generated a transgenic zebrafish model of neuroblastoma in which MYCN-induced tumors arise from a subpopulation of neuroblasts that migrate into the adrenal medulla analog following organogenesis. Coexpression of activated ALK with MYCN in this model triples the disease penetrance and markedly accelerates tumor onset. MYCN overexpression induces adrenal sympathetic neuroblast hyperplasia, blocks chromaffin cell differentiation, and ultimately triggers a developmentally-timed apoptotic response in the hyperplastic sympathoadrenal cells. Coexpression of activated ALK with MYCN provides prosurvival signals that block this apoptotic response and allow continued expansion and oncogenic transformation of hyperplastic neuroblasts, thus promoting progression to neuroblastoma.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
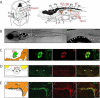
Figure 1. Transgenic gene expression in the sympathetic neurons and the interrenal gland
(A) Left: Schematic of a transverse section illustrating zebrafish anatomical structures, dorsal upwards. Right: Schematic of a sagittal section illustrating zebrafish anatomical structures, anterior to left. (B) EGFP expression (green) in the zebrafish chain of sympathetic ganglia (arrowheads), the IRG (arrow) and medulla oblongata (asterisk) at 3 wpf. Lateral view of confocal-brightfield image, anterior to left. The magnified view of the boxed region is shown on the right. Scale bar, 500 μm. (C) EGFP is coexpressed with TH in the SCG at 6 wpf (sagittal section); TH coexpression is indicated in red. Scale bar, 20 μm. (D) EGFP is coexpressed with TH in the chain of sympathetic ganglia at 6 wpf (transverse section). TH coexpression is indicated in red. Scale bar, 20 μm. (E) EGFP is coexpressed with TH in the IRG at 8 months postfertilization (mpf) (sagittal section). TH coexpression is indicated in red. Scale bar, 100 μm. Ao, aorta; Cb, cerebellum; Chfn, chromaffin cells; E, esophagus; G, gill; H, heart; HK, head kidney; I, intestine; IRG, interregnal gland; KB, kidney body; L, liver; Me, medulla; Nc, notochord; SB, swim bladder; SC, spinal cord; SCG, superior cervical ganglion; Sym.C, sympathetic chain. See also Figure S1.
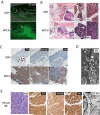
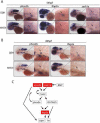
Figure 2. Neuroblastomas arise in _MYCN_-expressing transgenic zebrafish
(A) Top: DβH fish. Bottom: MYCN fish with EGFP-expressing tumor (arrow) at 32 weeks postfertilization (wpf). Scale bar, 1 mm. (B) Top: H&E-stained sagittal sections of DβH fish. Boxes indicate the SCG and IRG, and are magnified in the right panels. Bottom: H&E-stained sagittal sections of MYCN fish with neuroblastic tumors. Boxes indicate the SCG and IRG and are magnified in the right panels. Arrows indicate SCG neurons. The majority of tumors arise in the IRG, although as seen in this example, tumor cells in the SCG were occasionally observed in individual fish that also had tumors in the IRG. G, gill; L, liver; I, intestine; IRG, interregnal gland; SCG, superior cervical ganglion; T, testis. Scale bar, 50 μm. (C) Top: Sagittal sections through the interregnal gland of DβH fish. Chromaffin cells of the interregnal gland express TH (arrows). Bottom: Sagittal sections through the interregnal gland of a MYCN fish with EGFP-expressing tumor. Cells throughout the tumor in the interregnal gland express TH, Synaptophysin (Synap) and Hu. Scale bar, 100 μm. (D) Electron microscopy (EM) reveals neurosecretory granules in the _MYCN_-expressing tumors (arrows). Scale bar, 500 nm. (E) Pathological, immunohistochemical and ultrastructural analyses of a human neuroblastoma. Arrows point to neurosecretory granules. Scale bars, 500 μm (left panel), 100 μm (middle panels) and 2 μm (right panel), respectively. See also Figure S2.
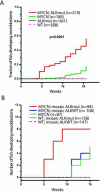
Figure 3. Activated ALK accelerates disease onset and increases the penetrance of MYCN-induced neuroblastoma
(A) Cumulative frequency of neuroblastoma in stable transgenic zebrafish by Kaplan-Meier analysis. ALKmut represents stable transgenic fish expressing the ALK (F1174L) transgene. WT, wild-type. (B) Onset of neuroblastoma in MYCN transgenic fish or wild-type (WT) fish as mosaics coinjected with the following DNA constructs: i) d_β_h-ALKF1174L and d_β_h-mCherry (mosaic ALKmut), ii) d_β_h-ALKWT and d_β_h-mCherry (mosaic ALKWT), or iii) d_β_h-mCherry alone. The difference between tumor onset by 9 wpf in the MYCN fish coinjected with d_β_h-ALKF1174L and d_β_h-mCherry (MYCN; mosaic ALKmut) and that in the MYCN line coinjected with d_β_h-ALKWT and d_β_h-mCherry (MYCN; mosaic ALKWT) or d_β_h-mCherry alone (MYCN) is significant at p=0.002 and p=0.007, respectively, with two-tailed Fisher exact test. See also Figure S3.
Figure 4. MYCN expression causes sympathoadrenal cell loss
(A) DβH transgenic line. Oblique views of d_β_h RNA expression (left panels); lateral views of EGFP expression in merged confocal-brightfield images (middle panels); dorsal views of th RNA expression (right panels). Arrows point to the SCG, and arrowheads point to the CG. Scale bar, 100 μm. (B) MYCN transgenic line. MYCN expression causes loss of cells in the SCG (arrows). Scale bar, 100 μm. (C) ALK transgenic line. ALK expression does not interfere with the SCG development (arrows). Scale bar, 100 μm. (D) MYCN;ALK transgenic line. Loss of cells in the SCG is not rescued by activated ALK expression (arrows). Scale bar, 100 μm. AAC, arch-associated catecholaminergic neurons; CG (arrowheads), cranial ganglia; DA, diencephalic dopaminergic neurons; e, ear; LC, locus coeruleus; MO, medulla oblongata; r, retina; SCG, superior cervical ganglion. See also Figure S4 and Table S1.
Figure 5. Expression of early sympathoadrenal markers is absent in MYCN transgenic embryos during early development
(A–B) Top panels: DβH; lower panels: MYCN transgenic fish. Expression of sympathoadrenal cell markers at 54 hpf (A) and 80 hpf (B). The magnified view of the boxed region is shown on the right. Arrows point to the superior cervical ganglion. Scale bars, 50 μm (left panels) and 100 μm (right panels, magnified view). (C) Diagram of the genetic interactions of sympathoadrenal genes during early development. Arrows indicate the activation of target genes. Curved arrows indicate positive feedback regulation. See also Figure S5.
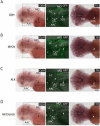
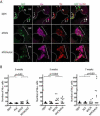
Figure 6. MYCN causes Hu+ cell hyperplasia in the interrenal gland
(A) Sagittal sections through the interrenal gland in DβH (top panels), MYCN (middle panels) and MYCN;ALK (lower panels) transgenic fish at 5wpf (dorsal up, anterior left). EGFP, green; Hu, magenta; TH, red. Representative sections through the interrenal gland in DβH fish contain 3–5 GFP+/Hu+/TH+ sympathetic neuroblasts (arrows) and many GFP+/Hu−/TH+ chromaffin cells (arrowheads). Hu+ cell numbers increase in MYCN and MYCN;ALK fish (brackets), and can be GFP+ and TH+. Dotted lines indicate the head kidney (HK) boundary. Scale bar, 20 μm. (B) Numbers of Hu+ interrenal gland cells in DβH, ALK, MYCN and MYCN;ALK transgenic fish at 3, 5 and 7 weeks. Means of Hu+ cell numbers were compared by the two-tailed Wilcoxon signed-rank test. See also Figure S6.
Figure 7. MYCN expression blocks chromaffin cell differentiation in the interrenal gland
(A–C) Numbers of GFP+/Hu− chromaffin cells in the interrenal gland in DβH, ALK, MYCN and MYCN;ALK transgenic fish at 3 weeks (A), 5 weeks (B) and 7 weeks (C). Means of GFP+/Hu− cell numbers were compared by the two-tailed Wilcoxon signed-rank test.
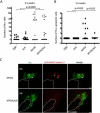
Figure 8. ALK inhibits a developmentally-timed apoptotic response triggered by MYCN overexpression in the interrenal gland
(A) Numbers of Hu+ interrenal gland cells in the DβH, ALK, MYCN and MYCN;ALK fish at 5.5 wpf. Means of Hu+ cell numbers were compared by the two-tailed Wilcoxon signed-rank test. (B) Numbers of apoptotic Hu+ interrenal gland cells in the DβH, ALK, MYCN and MYCN;ALK fish at 5.5 wpf. The numbers of transgenic fish at 5.5 wpf with apoptotic Hu+ cells in the interrenal gland were compared by two-tailed Fisher exact test. (C) Sagittal sections through the interrenal gland in MYCN (top panels) and MYCN;ALK (bottom panels) transgenic fish at 5.5 wpf (dorsal up, anterior left). Hu, green; activated Caspase-3, red. Hu+, activated Caspase-3+ apoptotic cells were detected in the MYCN transgenic fish (arrowheads). Dotted lines indicate the head kidney (HK) boundary. Scale bars, 10 μm. See also Figure S7.
Comment in
- ALK and MYCN: when two oncogenes are better than one.
Liu Z, Thiele CJ. Liu Z, et al. Cancer Cell. 2012 Mar 20;21(3):325-6. doi: 10.1016/j.ccr.2012.03.004. Cancer Cell. 2012. PMID: 22439928 Free PMC article.
Similar articles
- Neuroblastoma and Its Zebrafish Model.
Zhu S, Thomas Look A. Zhu S, et al. Adv Exp Med Biol. 2016;916:451-78. doi: 10.1007/978-3-319-30654-4_20. Adv Exp Med Biol. 2016. PMID: 27165366 Review. - Proliferation and Survival of Embryonic Sympathetic Neuroblasts by MYCN and Activated ALK Signaling.
Kramer M, Ribeiro D, Arsenian-Henriksson M, Deller T, Rohrer H. Kramer M, et al. J Neurosci. 2016 Oct 5;36(40):10425-10439. doi: 10.1523/JNEUROSCI.0183-16.2016. J Neurosci. 2016. PMID: 27707976 Free PMC article. - Anaplastic Lymphoma Kinase (ALK) regulates initiation of transcription of MYCN in neuroblastoma cells.
Schönherr C, Ruuth K, Kamaraj S, Wang CL, Yang HL, Combaret V, Djos A, Martinsson T, Christensen JG, Palmer RH, Hallberg B. Schönherr C, et al. Oncogene. 2012 Dec 13;31(50):5193-200. doi: 10.1038/onc.2012.12. Epub 2012 Jan 30. Oncogene. 2012. PMID: 22286764 - ALK is a MYCN target gene and regulates cell migration and invasion in neuroblastoma.
Hasan MK, Nafady A, Takatori A, Kishida S, Ohira M, Suenaga Y, Hossain S, Akter J, Ogura A, Nakamura Y, Kadomatsu K, Nakagawara A. Hasan MK, et al. Sci Rep. 2013 Dec 20;3:3450. doi: 10.1038/srep03450. Sci Rep. 2013. PMID: 24356251 Free PMC article. - New strategies in neuroblastoma: Therapeutic targeting of MYCN and ALK.
Barone G, Anderson J, Pearson AD, Petrie K, Chesler L. Barone G, et al. Clin Cancer Res. 2013 Nov 1;19(21):5814-21. doi: 10.1158/1078-0432.CCR-13-0680. Epub 2013 Aug 21. Clin Cancer Res. 2013. PMID: 23965898 Free PMC article. Review.
Cited by
- Expansion of a neural crest gene signature following ectopic MYCN expression in sympathoadrenal lineage cells in vivo.
Ibarra-García-Padilla R, Nambiar A, Hamre TA, Singleton EW, Uribe RA. Ibarra-García-Padilla R, et al. PLoS One. 2024 Sep 18;19(9):e0310727. doi: 10.1371/journal.pone.0310727. eCollection 2024. PLoS One. 2024. PMID: 39292691 Free PMC article. - Zebrafish as a Neuroblastoma Model: Progress Made, Promise for the Future.
Li S, Yeo KS, Levee TM, Howe CJ, Her ZP, Zhu S. Li S, et al. Cells. 2021 Mar 6;10(3):580. doi: 10.3390/cells10030580. Cells. 2021. PMID: 33800887 Free PMC article. Review. - Anaplastic lymphoma kinase is required for neurogenesis in the developing central nervous system of zebrafish.
Yao S, Cheng M, Zhang Q, Wasik M, Kelsh R, Winkler C. Yao S, et al. PLoS One. 2013 May 8;8(5):e63757. doi: 10.1371/journal.pone.0063757. Print 2013. PLoS One. 2013. PMID: 23667670 Free PMC article. - Integrative discovery of treatments for high-risk neuroblastoma.
Almstedt E, Elgendy R, Hekmati N, Rosén E, Wärn C, Olsen TK, Dyberg C, Doroszko M, Larsson I, Sundström A, Arsenian Henriksson M, Påhlman S, Bexell D, Vanlandewijck M, Kogner P, Jörnsten R, Krona C, Nelander S. Almstedt E, et al. Nat Commun. 2020 Jan 3;11(1):71. doi: 10.1038/s41467-019-13817-8. Nat Commun. 2020. PMID: 31900415 Free PMC article. - Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology.
Hallberg B, Palmer RH. Hallberg B, et al. Nat Rev Cancer. 2013 Oct;13(10):685-700. doi: 10.1038/nrc3580. Nat Rev Cancer. 2013. PMID: 24060861 Review.
References
- An M, Luo R, Henion PD. Differentiation and maturation of zebrafish dorsal root and sympathetic ganglion neurons. J Comp Neurol. 2002;446:267–275. - PubMed
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. - PubMed
- Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, Wang L, Soda M, Kikuchi A, Igarashi T, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 CA134743/CA/NCI NIH HHS/United States
- CA104605/CA/NCI NIH HHS/United States
- R00 NS058608/NS/NINDS NIH HHS/United States
- R01 CA104605/CA/NCI NIH HHS/United States
- K99CA134743/CA/NCI NIH HHS/United States
- R01 CA148688/CA/NCI NIH HHS/United States
- R00 CA134743/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases