β-Defensins: multifunctional modulators of infection, inflammation and more? - PubMed (original) (raw)
Review
β-Defensins: multifunctional modulators of infection, inflammation and more?
Fiona Semple et al. J Innate Immun. 2012.
Abstract
Defensins comprise one of the largest groups of host defence peptides, present throughout evolution, in fungi and flowering plants as well as in invertebrates and vertebrates. These cysteine-rich, cationic peptides have a common ability to kill a broad range of microorganisms including bacteria, yeast and viruses. As such, they are a strong component of the arsenal that is an organism's innate immunity. It is becoming increasingly clear, however, that antimicrobial action is only one of the numerous roles of these multifunctional peptides. In recent years, the functions of defensins in immunomodulation have been widely investigated, and their involvement in other processes (such as fertility) is becoming evident. This review addresses recent advances in the immunomodulatory activity of β-defensins as well as the involvement of β-defensins in fertility, development, wound healing and cancer.
Copyright © 2012 S. Karger AG, Basel.
Figures
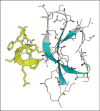
Fig. 1
β-Defensin structure. Homology model of β-defensin Defb14 based on the structure of hBD3, with antimicrobially potent residues 6–17 highlighted in yellow and β-sheets in blue [32].
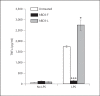
Fig. 2
Structural integrity is required for hBD3 immunosuppressive effect. Treatment of RAW264.7 macrophage cell line with LPS results in an increase in TNFα levels (measured by ELISA). In the presence of LPS, oxidized and canonically folded hBD3 (hBD3-F; black bars) inhibits this induction of TNFα. In contrast, linear hBD3 (hBD3-L) does not inhibit LPS-induced TNFα.
Fig. 3
hBD3 rapidly enters macrophages. The image shows a single cell (from the macrophage-like cell line RAW264.7) 10 min after the addition of hBD3 labeled with a TAMRA fluorochrome (red). Nuclear staining with DAPI (blue) demonstrates that at this time-point, hBD3 accumulates in the cytoplasm. Picture kindly provided by Dr. Heather MacPherson, MRC Human Genetics Unit, Edinburgh, UK.
Similar articles
- The immunology of host defence peptides: beyond antimicrobial activity.
Hancock RE, Haney EF, Gill EE. Hancock RE, et al. Nat Rev Immunol. 2016 May;16(5):321-34. doi: 10.1038/nri.2016.29. Epub 2016 Apr 18. Nat Rev Immunol. 2016. PMID: 27087664 Review. - Human defensins and cathelicidins in the skin: beyond direct antimicrobial properties.
Niyonsaba F, Nagaoka I, Ogawa H. Niyonsaba F, et al. Crit Rev Immunol. 2006;26(6):545-76. doi: 10.1615/critrevimmunol.v26.i6.60. Crit Rev Immunol. 2006. PMID: 17341194 Review. - [Characteristics of the main groups of human host-defensive peptides].
Lapis K. Lapis K. Orv Hetil. 2009 Jan 18;150(3):109-19. doi: 10.1556/OH.2009.28512. Orv Hetil. 2009. PMID: 19129146 Review. Hungarian. - Functions of Antimicrobial Peptides in Vertebrates.
Avila EE. Avila EE. Curr Protein Pept Sci. 2017;18(11):1098-1119. doi: 10.2174/1389203717666160813162629. Curr Protein Pept Sci. 2017. PMID: 27526932 Review. - Avian antimicrobial peptides: the defense role of beta-defensins.
Sugiarto H, Yu PL. Sugiarto H, et al. Biochem Biophys Res Commun. 2004 Oct 22;323(3):721-7. doi: 10.1016/j.bbrc.2004.08.162. Biochem Biophys Res Commun. 2004. PMID: 15381059 Review.
Cited by
- The Acari Hypothesis, V: deciphering allergenicity.
Retzinger AC, Retzinger GS. Retzinger AC, et al. Front Allergy. 2024 Nov 1;5:1454292. doi: 10.3389/falgy.2024.1454292. eCollection 2024. Front Allergy. 2024. PMID: 39552700 Free PMC article. - Impacts of Medications on Microbiome-mediated Protection against Enteric Pathogens.
Kumar A, Sun R, Habib B, Bencivenga-Barry NA, Ivanov II, Tamblyn R, Goodman AL. Kumar A, et al. Res Sq [Preprint]. 2024 Oct 18:rs.3.rs-5199936. doi: 10.21203/rs.3.rs-5199936/v1. Res Sq. 2024. PMID: 39483881 Free PMC article. Preprint. - Protective Capacity of Helichrysum italicum Infusion Against Intestinal Barrier Disruption and Translocation of Salmonella Infantis.
Kramberger K, Bezek Kranjc K, Jenko Pražnikar Z, Barlič-Maganja D, Kenig S. Kramberger K, et al. Pharmaceuticals (Basel). 2024 Oct 19;17(10):1398. doi: 10.3390/ph17101398. Pharmaceuticals (Basel). 2024. PMID: 39459037 Free PMC article. - Biologic Brachytherapy: Genetically Modified Surgical Flap as a Therapeutic Tool-A Systematic Review of Animal Studies.
Pascal W, Gotowiec M, Smoliński A, Suchecki M, Kopka M, Pascal AM, Włodarski PK. Pascal W, et al. Int J Mol Sci. 2024 Sep 25;25(19):10330. doi: 10.3390/ijms251910330. Int J Mol Sci. 2024. PMID: 39408659 Free PMC article. Review. - Senescence Rejuvenation through Reduction in Mitochondrial Reactive Oxygen Species Generation by Polygonum cuspidatum Extract: In Vitro Evidence.
Yoon JH, Kim YH, Jeong EY, Lee YH, Byun Y, Shin SS, Park JT. Yoon JH, et al. Antioxidants (Basel). 2024 Sep 14;13(9):1110. doi: 10.3390/antiox13091110. Antioxidants (Basel). 2024. PMID: 39334769 Free PMC article.
References
- Lynn DJ, Bradley DG. Discovery of alpha-defensins in basal mammals. Dev Comp Immunol. 2007;31:963–967. - PubMed
- Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2:727–738. - PubMed
- Nguyen TX, Cole AM, Lehrer RI. Evolution of primate theta-defensins: a serpentine path to a sweet tooth. Peptides. 2003;24:1647–1654. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials