Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis - PubMed (original) (raw)
Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis
Kerry Hunter et al. BMC Neurosci. 2012.
Abstract
Background: Type 2 diabetes is a risk factor for Alzheimer's disease (AD), most likely linked to an impairment of insulin signalling in the brain. Therefore, drugs that enhance insulin signalling may have therapeutic potential for AD. Liraglutide (Victoza) and exenatide (Byetta) are novel long-lasting analogues of the GLP-1 incretin hormone and are currently available to treat diabetes. They facilitate insulin signalling via the GLP-1 receptor (GLP-1R). Numerous in vitro and in vivo studies have shown that GLP-1 analogues have a range of neuroprotective properties. GLP-1Rs are expressed in the hippocampal area of the brain an important site of adult neurogenesis and maintenance of cognition and memory formation. Therefore, if GLP-1 analogues can cross the blood brain barrier, diffuse through the brain to reach the receptors and most importantly activate them, their neuroprotective effects may be realized.
Results: In the present study we profiled the GLP-1 receptor agonists liraglutide (Victoza) and lixisenatide (Lyxumia). We measured the kinetics of crossing the blood brain barrier (BBB), activation of the GLP-1R by measuring cAMP levels, and physiological effects in the brain on neuronal stem cell proliferation and neurogenesis. Both drugs were able to cross the BBB. Lixisenatide crossed the BBB at all doses tested (2.5, 25, or 250 nmol/kg bw ip.) when measured 30 min post-injection and at 2.5-25 nmol/kg bw ip. 3 h post-injection. Lixisenatide also enhanced neurogenesis in the brain. Liraglutide crossed the BBB at 25 and 250 nmol/kg ip. but no increase was detectable at 2.5 nmol/kg ip. 30 min post-injection, and at 250 nmol/kg ip. at 3 h post-injection. Liraglutide and lixisenatide enhanced cAMP levels in the brain, with lixisenatide being more effective.
Conclusions: Our results suggest that these novel incretin analogues cross the BBB and show physiological activity and neurogenesis in the brain, which may be of use as a treatment of neurodegenerative diseases.
Figures
Figure 1
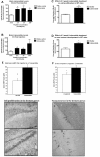
At 5 min post- ip. injection, there was no significant increase of liraglutide in the brains of mice (A). There was an increase in liraglutide levels in the brains of mice injected with 25 or 250 nmol/kg bw at 30 min (B) and only those injected with the highest dose at 3 h post-injection (C). Brain levels of cAMP were increased 30 min post injection with 25 nmol/kg bw ip.. * = p < 0.05. Values are the mean ± S.E.M.
Figure 2
(A) Lixisenatide levels in the brains at 30 min following i.p. (2.5, 25 or 250 nmol/kg body weight) injection were increased. (B) Lixisenatide levels in the brains at 3 h following i.p. injection were only increased for the lower doses (2.5, 25 nmol/kg body weight). (C) The number of neuronal progenitor cells in the dentate gyrus was increased after 3 weeks of ip injection 25 nmol/kg bw once-daily. (D) The number of young neurons in the dentate gyrus was also increased. (E) The level of cAMP was enhanced in the brains after injection with 25 nmol/kg bw ip. (F) When directly comparing the effects of liraglutide with lixisenatide, a significant difference between drugs is found.* = p < 0.05, ** = p < 0.01. Micrographs: BrdU stain: A Saline control B Lixisenatide treated, 3 weeks once daily i.p. injection. Blue arrows point to BrdU positively stained cells. DCX stain: Doublecortin stained immature neurons in dentate gyrus. A Saline control B Lixisenatide treated, 3 weeks once daily i.p. administration.
Similar articles
- Lixisenatide, a drug developed to treat type 2 diabetes, shows neuroprotective effects in a mouse model of Alzheimer's disease.
McClean PL, Hölscher C. McClean PL, et al. Neuropharmacology. 2014 Nov;86:241-58. doi: 10.1016/j.neuropharm.2014.07.015. Epub 2014 Aug 8. Neuropharmacology. 2014. PMID: 25107586 - Effects of the glucagon-like polypeptide-1 analogue (Val8)GLP-1 on learning, progenitor cell proliferation and neurogenesis in the C57B/16 mouse brain.
McGovern SF, Hunter K, Hölscher C. McGovern SF, et al. Brain Res. 2012 Sep 14;1473:204-13. doi: 10.1016/j.brainres.2012.07.029. Epub 2012 Jul 31. Brain Res. 2012. PMID: 22867941 - Novel GLP-1 mimetics developed to treat type 2 diabetes promote progenitor cell proliferation in the brain.
Hamilton A, Patterson S, Porter D, Gault VA, Holscher C. Hamilton A, et al. J Neurosci Res. 2011 Apr;89(4):481-9. doi: 10.1002/jnr.22565. Epub 2011 Jan 10. J Neurosci Res. 2011. PMID: 21312223 - Incretin analogues that have been developed to treat type 2 diabetes hold promise as a novel treatment strategy for Alzheimer's disease.
Holscher C. Holscher C. Recent Pat CNS Drug Discov. 2010 Jun;5(2):109-17. doi: 10.2174/157488910791213130. Recent Pat CNS Drug Discov. 2010. PMID: 20337586 Review. - Drugs developed for treatment of diabetes show protective effects in Alzheimer's and Parkinson's diseases.
Hölscher C. Hölscher C. Sheng Li Xue Bao. 2014 Oct 25;66(5):497-510. Sheng Li Xue Bao. 2014. PMID: 25331995 Review.
Cited by
- Glucagon-like peptide-1 (GLP-1) receptor agonists and neuroinflammation: Implications for neurodegenerative disease treatment.
Kopp KO, Glotfelty EJ, Li Y, Greig NH. Kopp KO, et al. Pharmacol Res. 2022 Dec;186:106550. doi: 10.1016/j.phrs.2022.106550. Epub 2022 Nov 11. Pharmacol Res. 2022. PMID: 36372278 Free PMC article. Review. - Liraglutide Reduces Alcohol Consumption, Anxiety, Memory Impairment, and Synapse Loss in Alcohol Dependent Mice.
Liu W, Wang Z, Wang W, Wang Z, Xing Y, Hölscher C. Liu W, et al. Neurochem Res. 2024 Apr;49(4):1061-1075. doi: 10.1007/s11064-023-04093-6. Epub 2024 Jan 24. Neurochem Res. 2024. PMID: 38267691 - IUPHAR review - Glucagon-like peptide-1 (GLP-1) and substance use disorders: An emerging pharmacotherapeutic target.
Bruns Vi N, Tressler EH, Vendruscolo LF, Leggio L, Farokhnia M. Bruns Vi N, et al. Pharmacol Res. 2024 Sep;207:107312. doi: 10.1016/j.phrs.2024.107312. Epub 2024 Jul 18. Pharmacol Res. 2024. PMID: 39032839 Review. - Neuroprotective effects of liraglutide for stroke model of rats.
Sato K, Kameda M, Yasuhara T, Agari T, Baba T, Wang F, Shinko A, Wakamori T, Toyoshima A, Takeuchi H, Sasaki T, Sasada S, Kondo A, Borlongan CV, Matsumae M, Date I. Sato K, et al. Int J Mol Sci. 2013 Oct 30;14(11):21513-24. doi: 10.3390/ijms141121513. Int J Mol Sci. 2013. PMID: 24177570 Free PMC article. - The novel GLP-1/GIP analogue DA5-CH reduces tau phosphorylation and normalizes theta rhythm in the icv. STZ rat model of AD.
Li C, Liu W, Li X, Zhang Z, Qi H, Liu S, Yan N, Xing Y, Hölscher C, Wang Z. Li C, et al. Brain Behav. 2020 Mar;10(3):e01505. doi: 10.1002/brb3.1505. Epub 2020 Jan 20. Brain Behav. 2020. PMID: 31960630 Free PMC article.
References
- Vilsboll T. Liraglutide: a new treatment for type 2 diabetes. Drugs Today (Barc) 2009;45(2):101–113. - PubMed
- Christensen M, Knop FK, Vilsboll T, Holst JJ. Lixisenatide for type 2 diabetis mellitus. Expert Opin Investig Drugs. 2011;20(4):549–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical