Activation of VTA GABA neurons disrupts reward consumption - PubMed (original) (raw)
Activation of VTA GABA neurons disrupts reward consumption
Ruud van Zessen et al. Neuron. 2012.
Abstract
The activity of ventral tegmental area (VTA) dopamine (DA) neurons promotes behavioral responses to rewards and environmental stimuli that predict them. VTA GABA inputs synapse directly onto DA neurons and may regulate DA neuronal activity to alter reward-related behaviors; however, the functional consequences of selective activation of VTA GABA neurons remains unknown. Here, we show that in vivo optogenetic activation of VTA GABA neurons disrupts reward consummatory behavior but not conditioned anticipatory behavior in response to reward-predictive cues. In addition, direct activation of VTA GABA projections to the nucleus accumbens (NAc) resulted in detectable GABA release but did not alter reward consumption. Furthermore, optogenetic stimulation of VTA GABA neurons directly suppressed the activity and excitability of neighboring DA neurons as well as the release of DA in the NAc, suggesting that the dynamic interplay between VTA DA and GABA neurons can control the initiation and termination of reward-related behaviors.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
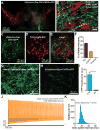
Figure 1. Optogenetic stimulation of VTA GABA neurons
(A) Expression of ChR2-eYFP following injection of the viral construct unilaterally into the VTA shown in green. Midbrain DA neurons, as indicated by tyrosine hydroxylase immunoreactivity, are shown in red. (B) Confocal compressed z-stack showing that ChR2-eYFP expressed in VTA fibers co-localizes with immunoreactivity for the vesicular GABA transporter. (C – F) Targeted eGFP expression in Cre expressing GABA neurons revealed minimal co-expression of TH in these neurons (n = 6 sections from n = 3 mice). (G,H) Confocal compressed z-stack showing ChR2-eYFP (green) in the VTA and Sn. DAPI counterstain shown in blue. (I) The average eYFP fluorescence intensity was significantly higher in the VTA compared to the Sn (t(10) = 6.18, p = 0.0001, n = 6 images per brain region from n = 6 mice). (J,K) Activation of ChR2 in VTA GABA neurons resulted in sustained high frequency activation during the 5 s stimulation (n = 4 neurons).
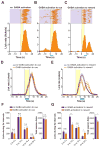
Figure 2. Optical activation of VTA GABA neurons timelocked to reward delivery disrupts reward consumption
(A) Example perievent histogram showing lick responses timelocked to reward-predictive cue (cue onset = 0 s; yellow bar) and reward delivery (occurring at t = 5 s) from a single mouse. Above raster plot indicates the licks made on each trial of the session (one trial per row). (B) Example perievent histogram showing data from a single mouse when VTA GABA neurons where activated (blue bar) during the reward predictive cue period (t = 0 – 5 s). (C) Example perievent histogram showing data from a single mouse when VTA GABA neurons where activated (blue bar) immediately following reward delivery (t = 5 – 10 s). (D) Average histogram data across all mice tested showing the change in the average lick rate when VTA GABA neurons where activated during cue presentation compared to sessions where mice did not receive optical stimulation. (E) Average histogram data across all mice tested showing the change in the average lick rate when VTA GABA neurons where activated immediately following reward delivery compared to sessions where mice did not receive optical stimulation. (F) Average data from (D) broken into 5 s time bins surrounding cue presentation (0 – 5 s) and reward consumption (5 – 10 s) periods (ANOVA for interaction: F(3,56) = 0.24, p = 0.86, n = 8 session per condition). Inset graph shows the average total session licks made during stimulation and non-stimulation sessions timelocked to cues (t(7) = 0.35, p = 0.73, n = 8 session per condition). (G) Average data from (E) broken into 5 s time bins surrounding cue presentation (0 – 5 s) and reward consumption (5 – 10 s) periods (ANOVA for interaction: F(3,56) = 5.4, p = 0.002, n = 8 session per condition). Inset graph shows the average total session licks made during stimulation and non-stimulation sessions timelocked to reward delivery (t(7) = 2.9, p = 0.02, n = 8 session per condition). Asterisks denote post-hoc statistical significance, * p < 0.05, ** p < 0.01.
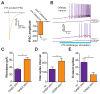
Figure 3. Optical activation VTA GABA neurons but not their projections to the NAc disrupt free-reward consumption
(A) Example raster plots showing free-reward licking immediately before, during, and after 5 s optical activation of VTA GABA neurons (blue bar). (B) Average data from all mice tested showing the number of licks made in the free reward consumption task surrounding the 5 s optical stimulation of VTA GABA neurons compared to blocked stimulation sessions (two-way ANOVA for a stimulation effect: F(1,30) = 7.89, p = 0.009, n = 6 mice). Asterisk denotes post-hoc statistical significance, p < 0.05. (C) Confocal image showing ChR2-eYFP expression in VTA GABAergic fibers innervating the NAc, DMS, and DLS (green). DAPI counterstain shown in blue. Average eYFP fluorescence intensity was significantly higher in the NAc compared to the DMS or DLS (F(2,29) = 8.31, p = 0.002, n = 10 images per brain region from n = 5 mice). Asterisk indicates p < 0.05. (D) Light evoked IPSCs via optical activation of VTA GABA fibers that innervate the NAc are blocked by bath application of 10 μM Gabazine (t(3) = 3.3, p = 0.04, n = 4 neurons). (E) Example raster plots showing free-reward licking immediately before, during, and after 5 s optical activation of VTA GABA fibers that project to the NAc. (F) Average data from all mice tested showing the number of licks made in the free reward consumption task surrounding the 5 s optical stimulation of VTA GABA projections to the NAc compared to blocked stimulation sessions (two-way ANOVA for a stimulation effect: F(1,30) = 0.01, p = 0.93, n = 6 mice).
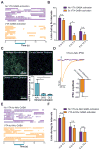
Figure 4. Activation of VTA GABA neurons reduces the excitability of VTA DA neurons and the release of DA in the NAc
(A) Light evoked IPSCs recorded from VTA DA neurons are blocked by bath application of 10 μM Gabazine (t(4) = 4.5, p = 0.01, n = 5 neurons). Asterisk indicates p < 0.05. (B) Current clamp recordings in DA neurons in response to +100 pA current injection ramps paired with a 5 s optical stimulation of VTA GABA neurons. The top trace shows the evoked firing in a neuron induced by the current injection; bottom trace shows the response in the same neuron when neighboring VTA GABA neurons are coincidentally activated. (C) VTA GABA activation decreases the excitability of VTA DA neurons as indicated by an increase in rheobase (t(5) = 5.2, p = 0.004, n = 6 neurons). Asterisk indicates p < 0.05. (D,E) VTA GABA activation decreases the activity of DA neurons as indicated by an increase in the inter-spike interval (t(5) = 4.4, p = 0.007, n = 6 neurons) and a decrease in the total number of evoked spikes (t(5) = 6.7, p = 0.001, n = 6 neurons). Asterisk indicates p < 0.05.
Figure 5. Activation of VTA GABA neurons reduces electrically evoked DA release in the NAc
(A) Voltammetric recordings indicated that activation of VTA GABA neurons reduces terminal DA release in the NAc induced by low frequency electrical stimulation of VTA. Representative concentration-time plots of electrically-evoked DA release measured in the NAc of anesthetized mice without (purple) and with (orange) optical stimulation of GABA neurons, which began 2.5 s prior to the VTA electrical stimulation and lasted for 2.5 s after. Arrowheads indicate the start of the electrical stimulation train. Inset shows background subtracted cyclic voltammograms, taken from the peaks of the signals, showing that the electrochemical signals were indicative of oxidized DA. (B) Voltammetric recordings with and without VTA GABA activation when the VTA is stimulated at a higher frequency. (C) Average data from all mice tested demonstrating frequency dependency of electrically-evoked DA release in the absence and presence of VTA GABA neuron activation (two-way ANOVA for a stimulation effect: F(1,28) = 43.01, p = 0.0001, n = 5 recording spots from n = 3 mice). Asterisks denote statistical significance (Bonferroni posttest, p < 0.05).
Similar articles
- GABA neurons of the VTA drive conditioned place aversion.
Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. Tan KR, et al. Neuron. 2012 Mar 22;73(6):1173-83. doi: 10.1016/j.neuron.2012.02.015. Epub 2012 Mar 21. Neuron. 2012. PMID: 22445344 Free PMC article. - Glutamatergic Ventral Pallidal Neurons Modulate Activity of the Habenula-Tegmental Circuitry and Constrain Reward Seeking.
Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz AV, Creed MC. Tooley J, et al. Biol Psychiatry. 2018 Jun 15;83(12):1012-1023. doi: 10.1016/j.biopsych.2018.01.003. Epub 2018 Jan 12. Biol Psychiatry. 2018. PMID: 29452828 Free PMC article. - Ventral tegmental area GABA neurons mediate stress-induced blunted reward-seeking in mice.
Lowes DC, Chamberlin LA, Kretsge LN, Holt ES, Abbas AI, Park AJ, Yusufova L, Bretton ZH, Firdous A, Enikolopov AG, Gordon JA, Harris AZ. Lowes DC, et al. Nat Commun. 2021 Jun 10;12(1):3539. doi: 10.1038/s41467-021-23906-2. Nat Commun. 2021. PMID: 34112787 Free PMC article. - VTA GABA Neurons at the Interface of Stress and Reward.
Bouarab C, Thompson B, Polter AM. Bouarab C, et al. Front Neural Circuits. 2019 Dec 5;13:78. doi: 10.3389/fncir.2019.00078. eCollection 2019. Front Neural Circuits. 2019. PMID: 31866835 Free PMC article. Review. - Progress in opioid reward research: From a canonical two-neuron hypothesis to two neural circuits.
Galaj E, Xi ZX. Galaj E, et al. Pharmacol Biochem Behav. 2021 Jan;200:173072. doi: 10.1016/j.pbb.2020.173072. Epub 2020 Nov 20. Pharmacol Biochem Behav. 2021. PMID: 33227308 Free PMC article. Review.
Cited by
- Pathophysiology, prognosis and treatment of tardive dyskinesia.
Takeuchi H, Mori Y, Tsutsumi Y. Takeuchi H, et al. Ther Adv Psychopharmacol. 2022 Oct 21;12:20451253221117313. doi: 10.1177/20451253221117313. eCollection 2022. Ther Adv Psychopharmacol. 2022. PMID: 36312846 Free PMC article. Review. - Distinct cell populations of ventral tegmental area process motivated behavior.
Kim MJ, Kaang BK. Kim MJ, et al. Korean J Physiol Pharmacol. 2022 Sep 1;26(5):307-312. doi: 10.4196/kjpp.2022.26.5.307. Korean J Physiol Pharmacol. 2022. PMID: 36039731 Free PMC article. Review. - Identification of rat ventral tegmental area GABAergic neurons.
Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Margolis EB, et al. PLoS One. 2012;7(7):e42365. doi: 10.1371/journal.pone.0042365. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860119 Free PMC article. - Neurochemical Signaling of Reward and Aversion to Ventral Tegmental Area Glutamate Neurons.
McGovern DJ, Polter AM, Root DH. McGovern DJ, et al. J Neurosci. 2021 Jun 23;41(25):5471-5486. doi: 10.1523/JNEUROSCI.1419-20.2021. Epub 2021 May 17. J Neurosci. 2021. PMID: 34001626 Free PMC article. - Terminal effects of optogenetic stimulation on dopamine dynamics in rat striatum.
Bass CE, Grinevich VP, Kulikova AD, Bonin KD, Budygin EA. Bass CE, et al. J Neurosci Methods. 2013 Apr 15;214(2):149-55. doi: 10.1016/j.jneumeth.2013.01.024. Epub 2013 Feb 4. J Neurosci Methods. 2013. PMID: 23391758 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- DA021634/DA/NIDA NIH HHS/United States
- DA029325/DA/NIDA NIH HHS/United States
- R21 DA029325/DA/NIDA NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- DA032750/DA/NIDA NIH HHS/United States
- K01 DA021634/DA/NIDA NIH HHS/United States
- R01 DA032750/DA/NIDA NIH HHS/United States
- R37 DA032750/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources