Sorafenib for treatment of hepatocellular carcinoma: a systematic review - PubMed (original) (raw)
Review
Sorafenib for treatment of hepatocellular carcinoma: a systematic review
Bingru Xie et al. Dig Dis Sci. 2012 May.
Abstract
Background: Sorafenib, a drug that inhibits Raf serine/threonine kinases mediating cell proliferation and receptor tyrosine kinases involved in angiogenesis, is approved for treatment of advanced hepatocellular carcinoma.
Aims: To explore the efficacy and safety of sorafenib for treating advanced HCC, and to identify clinical factors that might affect that efficacy and safety.
Methods: We conducted a systematic review using the PRISMA guidelines to identify prospective studies on sorafenib used alone or in combination with systemic and/or loco regional anti-tumor therapy for treating advanced HCC.
Results: We identified 21 prospective trials of sorafenib treatment alone (7) or combined with other treatment (14). In randomized, placebo-controlled trials, sorafenib prolonged overall survival by 2.3-2.8 months, extended the time to tumor progression by 1.4-2.7 months, and increased disease control by 11-19 %. OS and DCRs were lowest for studies with the highest percentage of hepatitis B patients. Most studies reported major side effects (diarrhea, fatigue, and hand-foot syndrome) in <15 % of patients, with greater incidence in patients with advanced cirrhosis and those treated with sorafenib in combination with 5-FU drugs.
Conclusions: Treatment with sorafenib results in statistically significant, but clinically modest, improvements in OS, TTP, and DCR. For patients with hepatitis B, response seems to be poorer than for those with hepatitis C. The frequency of hand-foot syndrome seems to be higher when sorafenib is used in advanced cirrhosis and is combined with 5-FU drugs. It is not clear that sorafenib combined with other treatments is more effective than sorafenib alone.
Conflict of interest statement
There is no financial or personal interest to report.
Figures
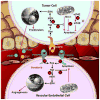
Figure 1
Sorafenib targets cell proliferation, apoptosis and angiogenesis. Red Xs indicate inhibition by sorafenib. Black arrows/lines indicate established pathways. Pink arrows/lines indicate proposed pathways affected by sorafenib. In tumor cells, sorafenib blocks the Raf/MEK/ERK pathway and induces apoptosis by inhibiting Raf isoforms. Sorafenib also appears to induce apoptosis through mechanisms independent of the Raf/MEK/ERK pathway including inhibition of eIF4E phosphorylation, and downregulation of Mcl-1. In vascular endothelial cells, sorafenib inhibits receptor tyrosine kinases involved in angiogenesis including VEGFR and PDGFR.
Figure 2
Trial selection flow
Similar articles
- Sorafenib: a review of its use in advanced hepatocellular carcinoma.
Keating GM, Santoro A. Keating GM, et al. Drugs. 2009;69(2):223-40. doi: 10.2165/00003495-200969020-00006. Drugs. 2009. PMID: 19228077 Review. - Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial.
Sansonno D, Lauletta G, Russi S, Conteduca V, Sansonno L, Dammacco F. Sansonno D, et al. Oncologist. 2012;17(3):359-66. doi: 10.1634/theoncologist.2011-0313. Epub 2012 Feb 14. Oncologist. 2012. PMID: 22334456 Free PMC article. Clinical Trial. - Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib.
Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M, Colucci G, Del Prete S, Tonini G. Vincenzi B, et al. Oncologist. 2010;15(1):85-92. doi: 10.1634/theoncologist.2009-0143. Epub 2010 Jan 5. Oncologist. 2010. PMID: 20051477 Free PMC article. - Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial.
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Cheng AL, et al. Lancet Oncol. 2009 Jan;10(1):25-34. doi: 10.1016/S1470-2045(08)70285-7. Epub 2008 Dec 16. Lancet Oncol. 2009. PMID: 19095497 Clinical Trial. - Sorafenib.
Rini BI. Rini BI. Expert Opin Pharmacother. 2006 Mar;7(4):453-61. doi: 10.1517/14656566.7.4.453. Expert Opin Pharmacother. 2006. PMID: 16503817 Review.
Cited by
- Role of antiviral therapy in reducing recurrence and improving survival in hepatitis B virus-associated hepatocellular carcinoma following curative resection (Review).
Zuo C, Xia M, Wu Q, Zhu H, Liu J, Liu C. Zuo C, et al. Oncol Lett. 2015 Feb;9(2):527-534. doi: 10.3892/ol.2014.2727. Epub 2014 Nov 21. Oncol Lett. 2015. PMID: 25624883 Free PMC article. - In a 'real-world', clinic-based community setting, sorafenib dose of 400 mg/day is as effective as standard dose of 800 mg/day in patients with advanced hepatocellular carcimona, with better tolerance and similar survival.
Shingina A, Hashim AM, Haque M, Suen M, Yoshida EM, Gill S, Donnellan F, Weiss AA. Shingina A, et al. Can J Gastroenterol. 2013 Jul;27(7):393-6. doi: 10.1155/2013/170546. Can J Gastroenterol. 2013. PMID: 23862169 Free PMC article. - Demonstration of the Antitumor Activity of the iNKT Agonist ABX196, a Novel Enhancer of Cancer Immunotherapy, in Melanoma and Hepatocarcinoma Mouse Models.
Scherrer D, Barrett N, Teyton L, Pearce T, Nitcheu J, Pouletty P, Santo J, Ehrlich HJ. Scherrer D, et al. Mol Cancer Ther. 2022 Dec 2;21(12):1788-1797. doi: 10.1158/1535-7163.MCT-22-0183. Mol Cancer Ther. 2022. PMID: 36198025 Free PMC article. - Multimodal treatment of hepatocellular carcinoma on cirrhosis: an update.
Vivarelli M, Montalti R, Risaliti A. Vivarelli M, et al. World J Gastroenterol. 2013 Nov 14;19(42):7316-26. doi: 10.3748/wjg.v19.i42.7316. World J Gastroenterol. 2013. PMID: 24259963 Free PMC article. Review. - Precision targeting in hepatocellular carcinoma: Exploring ligand-receptor mediated nanotherapy.
Zhou XQ, Li YP, Dang SS. Zhou XQ, et al. World J Hepatol. 2024 Feb 27;16(2):164-176. doi: 10.4254/wjh.v16.i2.164. World J Hepatol. 2024. PMID: 38495282 Free PMC article. Review.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
- Pons-Renedo F, Llovet JM. Hepatocellular carcinoma: a clinical update. MedGenMed. 2003;5:11. - PubMed
- Cancer Facts & Figures 2010. American Cancer Society; Atlanta, Geogia: 2010.
- El-Serag HB. Epidemiology of hepatocellular carcinoma in USA. Hepatol Res. 2007;37 (Suppl 2):S88–94. - PubMed
- El-Serag HB, Davila JA, Petersen NJ, et al. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817–23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous