Constraints imposed by the membrane selectively guide the alternating access dynamics of the glutamate transporter GltPh - PubMed (original) (raw)
Constraints imposed by the membrane selectively guide the alternating access dynamics of the glutamate transporter GltPh
Timothy R Lezon et al. Biophys J. 2012.
Abstract
Substrate transport in sodium-coupled amino acid symporters involves a large-scale conformational change that shifts the access to the substrate-binding site from one side of the membrane to the other. The structural change is particularly substantial and entails a unique piston-like quaternary rearrangement in glutamate transporters, as evidenced by the difference between the outward-facing and inward-facing structures resolved for the archaeal aspartate transporter Glt(Ph). These structural changes occur over time and length scales that extend beyond the reach of current fully atomic models, but are regularly explored with the use of elastic network models (ENMs). Despite their success with other membrane proteins, ENM-based approaches for exploring the collective dynamics of Glt(Ph) have fallen short of providing a plausible mechanism. This deficiency is attributed here to the anisotropic constraints imposed by the membrane, which are not incorporated into conventional ENMs. Here we employ two novel (to our knowledge) ENMs to demonstrate that one can largely capture the experimentally observed structural change using only the few lowest-energy modes of motion that are intrinsically accessible to the transporter, provided that the surrounding lipid molecules are incorporated into the ENM. The presence of the membrane reduces the overall energy of the transition compared with conventional models, showing that the membrane not only guides the selected mechanism but also acts as a facilitator. Finally, we show that the dynamics of Glt(Ph) is biased toward transitions of individual subunits of the trimer rather than cooperative transitions of all three subunits simultaneously, suggesting a mechanism of transport that exploits the intrinsic dynamics of individual subunits. Our software is available online at http://www.membranm.csb.pitt.edu.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
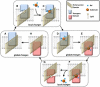
Figure 1
OF and IF structures of GltPh show a large displacement of the core domains. Each subunit of the homotrimer contains a trimerization domain (wheat) and a transport domain (blue and pink). (A) In the OF conformation (PDB code:
2NWL
), the transport domains extend into the EC medium, forming an aqueous basin around the trimer interface. (B) In the IF conformation (PDB code:
3KBC
), the transport domains are displaced across the membrane to the CP, whereas the trimer interface experiences little conformational change. The top diagrams in panels A and B represent the views from the EC region, and the lower diagrams display the side views within the membrane. Lipid molecules are represented by simple polar heads and hydrophobic tails. Molecular representations were rendered in VMD (47).
Figure 2
Many of the details of the GltPh transport cycle have been resolved. This cartoon summarizes the process for a single subunit. Starting from the OF unbound state (A), the flexible HP2 loop opens (B), exposing the sodium and substrate-binding sites. Three Na+ ions and the substrate bind (C), starting from the binding of a first Na+ (to the Na3 site), and then bind to a substrate and a sodium ion (to the Na2 site), and finally to the Na1 site. Here, for simplicity, we do not show these intermediate steps. The HP2 loop closes (D) and the transport domain moves through the membrane to the cytoplasm, placing the subunit in the IF closed state (E). The HP2 loop again opens (F), permitting the release of one Na+ ion from the Na1 site and subsequent hydration of the binding pocket. Water molecules aid in substrate dissociation by promoting the opening of HP1 and flooding the binding site, eventually leading to exit of substrate and the remaining Na+ ions (G). When the HP loops close (H), the transport domain moves back across the membrane to the EC face, completing the cycle. The global conformational changes D-E and G-A are the focus of this study.
Figure 3
First three modes of _im_ANM suggest a mechanism for conformational change. (A) The slowest nondegenerate ANM mode for the OF conformation of GltPh (top) is a pincer-type opening/closing of the trimer through motions of the transport domains. The same mode in the IF conformation (bottom) is a coordinated twisting of the transport domains. (B) In _im_ANM, the first nondegenerate mode is a piston-type axial motion of the transport domains for both the OF and IF conformations. It allows both the OF and IF conformations to move toward a transition intermediate, indicated by the arrows at far right. In all cases, the first twofold degenerate mode is the same as the mode shown, but with one of the subunits phase-shifted by 180°. (C) The transition for a single subunit. Starting from the OF crystal structure at far left, the motion along the first nondegenerate mode brings the transport domain into the membrane, as shown by the blue arrow. Starting from the IF crystal structure at right, the slowest nondegenerate mode moves the transport domain up toward the EC region. The change that is not captured by this mode is the difference between the two centermost structures. HP1 and HP2 are colored yellow and red, respectively.
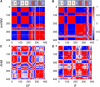
Figure 4
Separation of each subunit into transport and trimerization domains is visualized by using the cosines of the angles between residue motions (Eq. S4). Each matrix element indicates the cosine of the angle between motions of two residues, as calculated from the second mode of _im_ANM (A and B) or ANM (C and D). Red indicates comoving (fully correlated) residues, and blue indicates oppositely moving (fully anticorrelated) residues. (A) The second _im_ANM mode moves TM1, TM2, TM4, and TM5 in one direction, and TM3, TM6, TM7, HP2, and TM8 in the opposite direction. The coupling of TM3 and TM6 to the transport domain is contrary to the original domain definition provided by Boudker and co-workers (6). For reference, schematics of the locations of the eight TM helices are also shown, with the transport domain in blue, the trimerization domain in wheat, and the HP loops in dark red. (B) A similar sharp domain separation is seen for _im_ANM mode 2 of the IF conformation. (C and D) The ANM results produce a less clear demarcation.
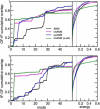
Figure 5
Inclusion of the membrane improves the efficiency of the modes in reproducing the structural deformation. In the left panels, the cumulative overlap of the deformation vector is plotted against the slowest 50 modes using different ENMs. The ANM (black) shows a steady increase in overlap with the number of modes considered. In contrast, the first nondegenerate mode of the _im_ANM (magenta) accounts for more than half of the deformation in either direction. The _ex_ANM (blue) shows behavior similar to that of the _im_ANM for the OF-IF transition, and ANM-like behavior for the IF-OF transition. A larger overlap can be obtained by increasing the _im_ANM membrane scaling factor, but this results in loss of overlap in higher modes (green). In the right panels, the cumulative overlap is plotted against the normalized energy of deformation between the two conformations. The membrane-augmented models (magenta and blue) allow for greater displacements at lower energies compared with ANM (black). The energetic cost of using the _im_ANM eventually overtakes that of ANM.
Figure 6
Cumulative overlap curves suggest possible transitions to intermediate conformations. Each plot shows the overlap of the first 20 modes of ANM (black), _im_ANM (magenta), and _ex_ANM (blue), with the deformation between the starting structure shown at left and the ending structure shown at top.
Similar articles
- Direct visualization of glutamate transporter elevator mechanism by high-speed AFM.
Ruan Y, Miyagi A, Wang X, Chami M, Boudker O, Scheuring S. Ruan Y, et al. Proc Natl Acad Sci U S A. 2017 Feb 14;114(7):1584-1588. doi: 10.1073/pnas.1616413114. Epub 2017 Jan 30. Proc Natl Acad Sci U S A. 2017. PMID: 28137870 Free PMC article. - Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats.
Crisman TJ, Qu S, Kanner BI, Forrest LR. Crisman TJ, et al. Proc Natl Acad Sci U S A. 2009 Dec 8;106(49):20752-7. doi: 10.1073/pnas.0908570106. Epub 2009 Nov 19. Proc Natl Acad Sci U S A. 2009. PMID: 19926849 Free PMC article. - Transport rates of a glutamate transporter homologue are influenced by the lipid bilayer.
McIlwain BC, Vandenberg RJ, Ryan RM. McIlwain BC, et al. J Biol Chem. 2015 Apr 10;290(15):9780-8. doi: 10.1074/jbc.M114.630590. Epub 2015 Feb 20. J Biol Chem. 2015. PMID: 25713135 Free PMC article. - Transport mechanism of a glutamate transporter homologue GltPh.
Ji Y, Postis VL, Wang Y, Bartlam M, Goldman A. Ji Y, et al. Biochem Soc Trans. 2016 Jun 15;44(3):898-904. doi: 10.1042/BST20160055. Biochem Soc Trans. 2016. PMID: 27284058 Free PMC article. Review. - The twisting elevator mechanism of glutamate transporters reveals the structural basis for the dual transport-channel functions.
Chen I, Wu Q, Font J, Ryan RM. Chen I, et al. Curr Opin Struct Biol. 2022 Aug;75:102405. doi: 10.1016/j.sbi.2022.102405. Epub 2022 Jun 13. Curr Opin Struct Biol. 2022. PMID: 35709614 Review.
Cited by
- Finely ordered intracellular domain harbors an allosteric site to modulate physiopathological function of P2X3 receptors.
Lin YY, Lu Y, Li CY, Ma XF, Shao MQ, Gao YH, Zhang YQ, Jiang HN, Liu Y, Yang Y, Huang LD, Cao P, Wang HS, Wang J, Yu Y. Lin YY, et al. Nat Commun. 2024 Sep 3;15(1):7652. doi: 10.1038/s41467-024-51815-7. Nat Commun. 2024. PMID: 39227563 Free PMC article. - Data-Driven Ensemble Docking to Map Molecular Interactions of Steroid Analogs with Hepatic Organic Anion Transporting Polypeptides.
Tuerkova A, Ungvári O, Laczkó-Rigó R, Mernyák E, Szakács G, Özvegy-Laczka C, Zdrazil B. Tuerkova A, et al. J Chem Inf Model. 2021 Jun 28;61(6):3109-3127. doi: 10.1021/acs.jcim.1c00362. Epub 2021 Jun 9. J Chem Inf Model. 2021. PMID: 34105971 Free PMC article. - Coupling between neurotransmitter translocation and protonation state of a titratable residue during Na ⁺-coupled transport.
Bahar I. Bahar I. Biophys J. 2014 Jun 17;106(12):2547-8. doi: 10.1016/j.bpj.2014.05.011. Biophys J. 2014. PMID: 24940770 Free PMC article. No abstract available. - Not just an oil slick: how the energetics of protein-membrane interactions impacts the function and organization of transmembrane proteins.
Mondal S, Khelashvili G, Weinstein H. Mondal S, et al. Biophys J. 2014 Jun 3;106(11):2305-16. doi: 10.1016/j.bpj.2014.04.032. Biophys J. 2014. PMID: 24896109 Free PMC article. Review. - Structure of a bacterial energy-coupling factor transporter.
Wang T, Fu G, Pan X, Wu J, Gong X, Wang J, Shi Y. Wang T, et al. Nature. 2013 May 9;497(7448):272-6. doi: 10.1038/nature12045. Epub 2013 Apr 14. Nature. 2013. PMID: 23584587
References
- Tanaka K., Watase K., Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. - PubMed
- Zerangue N., Kavanaugh M.P. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. - PubMed
- Amara S.G., Fontana A.C. Excitatory amino acid transporters: keeping up with glutamate. Neurochem. Int. 2002;41:313–318. - PubMed
- Groeneveld M., Slotboom D.-J. Na(+):aspartate coupling stoichiometry in the glutamate transporter homologue Glt(Ph) Biochemistry. 2010;49:3511–3513. - PubMed
- Yernool D., Boudker O., Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000005/TR/NCATS NIH HHS/United States
- U54 GM087519/GM/NIGMS NIH HHS/United States
- R01 GM086238/GM/NIGMS NIH HHS/United States
- UL1 RR024153/RR/NCRR NIH HHS/United States
- 5R01 GM086238-03/GM/NIGMS NIH HHS/United States
- 1U54GM087519-01A1/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources