Thioredoxin-1 regulates cellular heme insertion by controlling S-nitrosation of glyceraldehyde-3-phosphate dehydrogenase - PubMed (original) (raw)
Thioredoxin-1 regulates cellular heme insertion by controlling S-nitrosation of glyceraldehyde-3-phosphate dehydrogenase
Ritu Chakravarti et al. J Biol Chem. 2012.
Abstract
NO generated by inducible NOS (iNOS) causes buildup of S-nitrosated GAPDH (SNO-GAPDH) in cells, which then inhibits further iNOS maturation by limiting the heme insertion step (Chakravarti, R., Aulak, K. S., Fox, P. L., and Stuehr, D. J. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 18004-18009). We investigated what regulates this process utilizing a slow-release NO donor (NOC-18) and studying changes in cellular SNO-GAPDH levels during and after NO exposure. Culturing macrophage-like cells with NOC-18 during cytokine activation caused buildup of heme-free (apo) iNOS and SNO-GAPDH. Upon NOC-18 removal, the cells quickly recovered their heme insertion capacity in association with rapid SNO-GAPDH denitrosation, implying that these processes are linked. We then altered cell expression of thioredoxin-1 (Trx1) or S-nitrosoglutathione reductase, both of which can function as a protein denitrosylase. Trx1 knockdown increased SNO-GAPDH levels in cells, made heme insertion hypersensitive to NO, and increased the recovery time, whereas Trx1 overexpression greatly diminished SNO-GAPDH buildup and protected heme insertion from NO inhibition. In contrast, knockdown of S-nitrosoglutathione reductase expression had little effect on these parameters. Experiments utilizing C152S GAPDH confirmed that the NO effects are all linked to S-nitrosation of GAPDH at Cys-152. We conclude (i) that NO inhibition of heme insertion and its recovery can be rapid and dynamic processes and are inversely linked to the S-nitrosation of GAPDH and (ii) that the NO sensitivity of heme insertion can vary depending on the Trx1 expression level due to Trx1 acting as an SNO-GAPDH denitrosylase. Together, our results identify a new way that cells regulate heme protein maturation during inflammation.
Figures
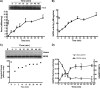
FIGURE 1.
Recovery of cellular heme insertion and change in SNO-GAPDH level after removing NO donor. Cells were activated for 17 h to express iNOS in the presence or absence of 250 μ
m
NOC-18. NOC-18 was removed and replaced with medium containing
l
-NAME and cycloheximide to initiate recovery of heme insertion, with cells frozen and supernatants created at the indicated times. A, heme content of iNOS determined spectroscopically after adding dithionite and CO to supernatants from cells that had (■) or had not (□) received the NO donor. Western analysis was performed to compare the total iNOS protein in each supernatant sample (inset). B, iNOS NO synthesis activity measured in the supernatants. C, upper panel, in-gel heme staining of the iNOS protein bands versus time in supernatants from cells that did or did not (R) receive the NO donor, along with Western analysis of the total iNOS protein in the supernatants. Lower panel, heme stain band densities (upper panel) as quantified using ImageJ. D, GAPDH enzymatic activity (□) and relative SNO-GAPDH level (▴) versus time in supernatants from cells that had received the NO donor. In A, B, and D, the values are the mean ± S.D. of at least three independent experiments.
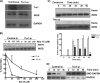
FIGURE 2.
Knockdown of Trx1 expression makes heme insertion more sensitive to NO inhibition. RAW 264.7 cells were transfected with Trx1 (Trx1-si, Trx-si) or control (Control-si, C-si, Cntl-si) siRNA and cytokine-induced for 17 h to express iNOS in the presence
l
-NAME and the indicated concentrations of NOC-18. The cells were then harvested and analyzed (A and B), or NOC-18 (250 μ
m
) was removed, followed by additional culture for various times with
l
-NAME before harvest (C and D). A, Western analysis of Trx1, GAPDH, and iNOS expression in the knockdown versus control cell supernatants at 17 h. B, upper panel, in-gel heme staining of the iNOS protein bands from cell supernatants and their corresponding total iNOS levels at 17 h as determined by Western analysis. Lower panel, relative intensities of the heme stain bands determined using Image J (mean ± S.D. of three independent experiments). C, upper and middle panels, in-gel heme staining of the iNOS protein bands in the supernatants versus recovery time (+
l
-NAME) from cells that had received either control or Trx1 siRNA as indicated and 250 μ
m
NOC-18 during 17 h of induction. Lower panel, heme stain band densities as quantified using ImageJ (mean ± S.D. of at least three independent experiments). D, relative SNO-GAPDH (biotin switch assay) and total GAPDH (Western blotting) present in supernatant samples taken at the indicated times during the recovery period.
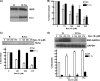
FIGURE 3.
Trx1 overexpression protects heme insertion from NO inhibition. RAW 264.7 cells were transfected to overexpress Trx1 (R-Trx) or the vector control (R) and then induced for 17 h to express iNOS in the presence of
l
-NAME and the indicated NOC-18 concentrations, and cell supernatants were prepared and analyzed. A, Western analysis showing expression levels of Trx1 and the total iNOS protein. B, spectroscopically determined iNOS heme content (normalized to 0 [NOC-18] = 100%) of supernatant samples of equal total protein. C, in-gel heme stain of the iNOS bands (upper panel) and relative intensities of the heme stain iNOS bands (lower panel). D, levels of SNO-GAPDH determined by biotin switch assay and total GAPDH determined by Western blotting in samples of equivalent total protein from the cell supernatants (upper panel) and the relative intensities of the SNO-GAPDH bands (lower panels). In B–D, values shown are the average of three independent experiments in each case.
FIGURE 4.
GSNOR knockdown has minor impacts. RAW 264.7 cells were transfected with control (Control-si) or GSNOR (GSNOR-si) siRNA and cytokine-induced for 17 h to express iNOS in the presence of
l
-NAME and the indicated concentrations of NOC-18. The cells were then harvested and analyzed, or NOC-18 (250 μ
m
) was removed, followed by further culture for various times with
l
-NAME before harvest. A, upper panel, Western analysis of iNOS and GSNOR expression in control versus knockdown cell supernatants created at the indicated times during the recovery period. Lower panel, heme stain band intensities from the cell supernatants. Relative intensities were determined using Image J (mean ± S.D. of three independent experiments). B, levels of SNO-GAPDH and total GAPDH in the supernatants at the indicated recovery times. C, upper panel, in-gel heme staining of iNOS protein bands and total iNOS (Western blotting) in the supernatants at 17 h from cells receiving the indicated NOC-18 concentrations. Lower panel, heme stain band densities as quantified using ImageJ (mean ± S.D. of at least three independent experiments).
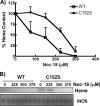
FIGURE 5.
C152S GAPDH decreases NO sensitivity of heme insertion. Cells overexpressing either wild-type or C152S GAPDH were induced for 17 h to express iNOS in the presence of the indicated NOC-18 concentrations, and cell supernatants were prepared and analyzed. A, spectroscopically determined iNOS heme content (normalized to 0 [NOC-18] = 100%) in samples of equal total protein from each supernatant. Data are the mean ± S.D. of three independent experiments. B, relative heme content of iNOS (upper panel; in-gel heme staining) and Western analysis of the total iNOS protein (lower panel) in the supernatant samples (equal total protein loaded). Data are representative of three similar experiments.
Similar articles
- GAPDH regulates cellular heme insertion into inducible nitric oxide synthase.
Chakravarti R, Aulak KS, Fox PL, Stuehr DJ. Chakravarti R, et al. Proc Natl Acad Sci U S A. 2010 Oct 19;107(42):18004-9. doi: 10.1073/pnas.1008133107. Epub 2010 Oct 4. Proc Natl Acad Sci U S A. 2010. PMID: 20921417 Free PMC article. - GAPDH S-nitrosation contributes to age-related sarcopenia through mediating apoptosis.
Xie T, Qiao X, Sun C, Chu B, Meng J, Chen C. Xie T, et al. Nitric Oxide. 2022 Mar 1;120:1-8. doi: 10.1016/j.niox.2021.12.006. Epub 2021 Dec 29. Nitric Oxide. 2022. PMID: 34973445 - Heme binding properties of glyceraldehyde-3-phosphate dehydrogenase.
Hannibal L, Collins D, Brassard J, Chakravarti R, Vempati R, Dorlet P, Santolini J, Dawson JH, Stuehr DJ. Hannibal L, et al. Biochemistry. 2012 Oct 30;51(43):8514-29. doi: 10.1021/bi300863a. Epub 2012 Oct 15. Biochemistry. 2012. PMID: 22957700 Free PMC article. - New roles for GAPDH, Hsp90, and NO in regulating heme allocation and hemeprotein function in mammals.
Stuehr DJ, Dai Y, Biswas P, Sweeny EA, Ghosh A. Stuehr DJ, et al. Biol Chem. 2022 Sep 26;403(11-12):1005-1015. doi: 10.1515/hsz-2022-0197. Print 2022 Nov 25. Biol Chem. 2022. PMID: 36152339 Free PMC article. Review. - The role of posttranslational modification in moonlighting glyceraldehyde-3-phosphate dehydrogenase structure and function.
Sirover MA. Sirover MA. Amino Acids. 2021 Apr;53(4):507-515. doi: 10.1007/s00726-021-02959-z. Epub 2021 Mar 2. Amino Acids. 2021. PMID: 33651246 Review.
Cited by
- A bipartite interaction between Hsp70 and CHIP regulates ubiquitination of chaperoned client proteins.
Zhang H, Amick J, Chakravarti R, Santarriaga S, Schlanger S, McGlone C, Dare M, Nix JC, Scaglione KM, Stuehr DJ, Misra S, Page RC. Zhang H, et al. Structure. 2015 Mar 3;23(3):472-482. doi: 10.1016/j.str.2015.01.003. Epub 2015 Feb 12. Structure. 2015. PMID: 25684577 Free PMC article. - Thioredoxin 1 promotes autophagy through transnitrosylation of Atg7 during myocardial ischemia.
Nagarajan N, Oka SI, Nah J, Wu C, Zhai P, Mukai R, Xu X, Kashyap S, Huang CY, Sung EA, Mizushima W, Titus AS, Takayama K, Mourad Y, Francisco J, Liu T, Chen T, Li H, Sadoshima J. Nagarajan N, et al. J Clin Invest. 2023 Feb 1;133(3):e162326. doi: 10.1172/JCI162326. J Clin Invest. 2023. PMID: 36480290 Free PMC article. - Post-Translational Modifications to Cysteine Residues in Plant Proteins and Their Impact on the Regulation of Metabolism and Signal Transduction.
Boutin C, Clément C, Rivoal J. Boutin C, et al. Int J Mol Sci. 2024 Sep 12;25(18):9845. doi: 10.3390/ijms25189845. Int J Mol Sci. 2024. PMID: 39337338 Free PMC article. Review. - Glyceraldehyde-3-phosphate Dehydrogenase is a Multifaceted Therapeutic Target.
Lazarev VF, Guzhova IV, Margulis BA. Lazarev VF, et al. Pharmaceutics. 2020 May 2;12(5):416. doi: 10.3390/pharmaceutics12050416. Pharmaceutics. 2020. PMID: 32370188 Free PMC article. Review. - Paradoxical Roles of Antioxidant Enzymes: Basic Mechanisms and Health Implications.
Lei XG, Zhu JH, Cheng WH, Bao Y, Ho YS, Reddi AR, Holmgren A, Arnér ES. Lei XG, et al. Physiol Rev. 2016 Jan;96(1):307-64. doi: 10.1152/physrev.00010.2014. Physiol Rev. 2016. PMID: 26681794 Free PMC article. Review.
References
- Benhar M., Forrester M. T., Stamler J. S. (2009) Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 10, 721–732 - PubMed
- Guikema B., Lu Q., Jourd'heuil D. (2005) Chemical considerations and biological selectivity of protein nitrosation: implications for NO-mediated signal transduction. Antioxid. Redox Signal. 7, 593–606 - PubMed
- Thomas D. D., Ridnour L. A., Isenberg J. S., Flores-Santana W., Switzer C. H., Donzelli S., Hussain P., Vecoli C., Paolocci N., Ambs S., Colton C. A., Harris C. C., Roberts D. D., Wink D. A. (2008) The chemical biology of nitric oxide: implications in cellular signaling. Free Radic. Biol. Med. 45, 18–31 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials