Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3 - PubMed (original) (raw)
Kindlins, integrin activation and the regulation of talin recruitment to αIIbβ3
Bryan N Kahner et al. PLoS One. 2012.
Abstract
Talins and kindlins bind to the integrin β3 cytoplasmic tail and both are required for effective activation of integrin αIIbβ3 and resulting high-affinity ligand binding in platelets. However, binding of the talin head domain alone to β3 is sufficient to activate purified integrin αIIbβ3 in vitro. Since talin is localized to the cytoplasm of unstimulated platelets, its re-localization to the plasma membrane and to the integrin is required for activation. Here we explored the mechanism whereby kindlins function as integrin co-activators. To test whether kindlins regulate talin recruitment to plasma membranes and to αIIbβ3, full-length talin and kindlin recruitment to β3 was studied using a reconstructed CHO cell model system that recapitulates agonist-induced αIIbβ3 activation. Over-expression of kindlin-2, the endogenous kindlin isoform in CHO cells, promoted PAR1-mediated and talin-dependent ligand binding. In contrast, shRNA knockdown of kindlin-2 inhibited ligand binding. However, depletion of kindlin-2 by shRNA did not affect talin recruitment to the plasma membrane, as assessed by sub-cellular fractionation, and neither over-expression of kindlins nor depletion of kindlin-2 affected talin interaction with αIIbβ3 in living cells, as monitored by bimolecular fluorescence complementation. Furthermore, talin failed to promote kindlin-2 association with αIIbβ3 in CHO cells. In addition, purified talin and kindlin-3, the kindlin isoform expressed in platelets, failed to promote each other's binding to the β3 cytoplasmic tail in vitro. Thus, kindlins do not promote initial talin recruitment to αIIbβ3, suggesting that they co-activate integrin through a mechanism independent of recruitment.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Model of agonist-induced αIIbβ3 activation.
(A) Stimulation of a platelet agonist receptor (e.g., PAR1) by an agonist leads to the activation of Rap1, resulting in targeting of its effector, RIAM, to the plasma membrane. (B) Cell stimulation also releases talin from its auto-inhibitory state, resulting in separation of the THD from the talin rod domain and recruitment of talin to the membrane-bound Rap1/RIAM complex. (C) Membrane-bound talin is recruited to αIIbβ3 by interaction of the THD with membrane-distal residues in the β3 cytoplasmic domain. (D) Further interactions of the THD with membrane-proximal β3 tail residues and membrane phospholipids leads to separation of the αIIb and β3 tail and transmembrane domains, triggering propagated changes in the extracellular domains leading to high-affinity binding of adhesive ligands, such as fibrinogen. While kindlins, like talin, can interact with the β3 cytoplasmic tail, they can also bind to other proteins , , and the molecular basis of their integrin co-activating function remains unclear. This working model is based on published studies summarized in .
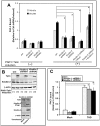
Figure 2. Kindlin-2 requirement for talin-dependent, agonist-induced αIIbβ3 activation in CHO cells.
(A) Agonist-induced PAC-1 binding determined in kindlin-2 knockdown cells. αIIbβ3 CHO cells engineered to conditionally express PAR1 and talin were transduced with lentivirus encoding control (Ctrl) or kindlin-2 shRNAs as described in Experimental Procedures. Cells were incubated for 20 min at room temperature with 100 µM SFLLRN (or vehicle), and specific PAC-1 binding was quantified by flow cytometry as described . To control for off target effects of knock-down constructs, shRNA-transduced cells were transiently co-transfected with an shRNA-resistant form of Flag-kindlin-2 (or empty vector, Mock) and Tac as a transfection marker. After induction of PAR1 and talin with doxycycline, specific PAC-1 binding was measured and normalized to integrin expression as determined by D57 staining. For clarity, data are expressed as the fold-increase in PAC-1 binding relative to binding observed with doxycycline-induced cells transduced with control shRNA. Data represent means ± SEM of six independent experiments (asterisk, P<0.01). (B) western blots were performed to assess expression of talin and kindlin-2 in lysates of cells studied in panel A. β-actin was monitored as a loading control. In the kindlin-2 rescue experiments, kindlin-2 was assessed both with an antibody to kindlin-2 and an antibody to the Flag epitope. The cell lysates were from both uninfected and virus transduced cells whereas using flow cytometry gating, only virus transduced cells were analyzed in panel A. (C) Kindlin-2 shRNA has no effect on PAC-1 binding induced by THD. αIIbβ3 CHO cells were transduced with lentivirus encoding _kindlin_-2 (or control) shRNA. Cells were transfected as indicated with THD, empty vector (Mock) and DsRed. PAC-1 binding to transfected cells was quantified by flow cytometry. PAC1 binding was normalized to PAC1 binding when integrins are fully activated by an activating antibody, which also is sensitive to the integrin expression level . For clarity, data are expressed as the fold-increase in PAC-1 binding relative to binding observed with THD transfected/control shRNA transduced cells. Data represent means ± SEM of 7 experiments. (Asterisk, P<0.01 against mock transfected/control shRNA transduced cells).
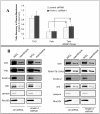
Figure 3. Effect of kindlin-2 knockdown on talin recruitment to the membrane.
(A) αIIβb3 CHO cells were transduced with lentivirus encoding either control shRNA or _kindlin_-2 shRNA. Cells were then transfected as indicated with THD, talin, or talin and RIAM1-176CAAX. Intact cells were surface biotinylated in order to isolate membrane-bound proteins. Cells were broken-up by shear and then underwent serial centrifugation to isolate the nuclear/intact cell fraction, cytosolic fraction and crude membranes. The crude membranes were further purified with streptavidin conjugated beads. The streptavidin bound material was isolated as the plasma membrane fraction. The amount of THD and talin in each fraction as well as in whole cell lysate (WCL) was quantified by western blot. Data is expressed as relative protein recovery normalized to the recovery of integrin αIIb subunit in plasma membrane. Data represent means ± SEM of three independent experiments (asterisk, P<0.10 in paired t-test). (B) Representative western blots of the subcellular fractionation experiments showing the WCL and plasma membrane fraction. Western blot of the WCL showed that αIIb expression levels are unchanged. RhoGDI serve both as a loading control , and a cytosolic marker. Western blots of each target proteins and markers were cut and juxtaposed for clarity. The complete blot images with all the subcellular fractions are shown in Figure S3.
Figure 4. Effect of kindlin over-expression or depletion on talin interaction with αIIbβ3 in living cells and in vitro.
(A) Schematic illustration of BiFC in CHO cells depicting αIIb-VC, VN-talin, and β3. When VN-talin interacts with αIIb-VCβ3 through the β3 tail, VN and VC should reconstitute the Venus fluorophore, resulting in BiFC . (B) Neither over-expression nor knock-down of kindlins promote talin interaction with αIIbβ3. αIIb-VCβ3 CHO cells (or cells expressing mutant αIIb-VCβ3Δ724) were co-transfected with an expression vector for Flag-kindlin-2, Flag-kindlin-3, or empty vector (Mock) along with Tac as a transfection marker. To assess the effects of kindlin-2 knockdown, αIIb-VCβ3 CHO cells were transduced with _kindlin_-2 shRNA1 (or control shRNA, Mock). VN-talin expression was induced with doxycycline, and BiFC was quantified by flow cytometry. BiFC fluorescence was normalized to αIIbβ3 expression and presented as fold-increase relative to doxycycline-induced, mock-treated cells. Data represent means ± SEM of three experiments (asterisk denotes statistically significant difference against mock/induced, P<0.05). (C) Western blots were performed to monitor expression of talin and kindlins in lysates of αIIb-VCβ3 CHO cells studied in panel B. Left side: western blot of WCL showed successful expression of kindlin-2, kindlin-3 and VN-talin. Right side: Western blots of WCL showed successful knockdown of kindlin-2 by shRNA1, without effects on VN-talin expression. (D) Kindlin-2 knockdown decreased basal PAC-1 binding as expected (asterisk, P<0.01). (E) Purified talin with or without addition of kindlin-3 was incubated with the recombinant β3 cytoplasmic tails conjugated to neutravidin beads as described in Experimental Procedures. After washing, proteins bound to the beads were detected on western blots. Band intensities were quantified in LICOR, normalized to talin binding in the absence of kinldin-3, and presented as a curve. Insert shows a representative western blot of 3 independent experiments. Increasing amounts of β3 tail-bound kindlin-3 failed to promote β3–talin interaction. Data represent means ± SEM of three experiments. (F) Same as (E) but purified THD was used instead of talin. Increasing amount of β3 tail-bound kindlin-3 failed to promote β3–THD interaction. Blots were performed with anti-talin (8d4) for talin, anti-flag and anti-His6 for kindlin-3 and THD. β3 tail loading was visualized by Coomassie stain. (asterisks in E and F denote statistically significant differences compared to talin or THD binding in the absence of kindlin-3, P<0.05).
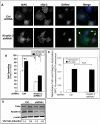
Figure 5. Subcellular localization of BiFC signals.
αIIb-VCβ3 CHO cells were transduced with _kindlin_-2 (or control) shRNA lentiviruses also encoding DsRed, and VN-talin was induced by doxycycline. (A) Cells were incubated on fibrinogen-coated plates (100 µg/ml coating concentration) for 45 min, fixed, stained with antibody D57 for αIIbβ3, and examined by deconvolution microscopy (BiFC: Green, αIIbβ3: blue, Transduced: red). The arrows point to transduced cells, and the arrowhead to a non-transduced cell. (B) Spreading of transduced cells was examined and data were expressed as mean cell surface areas measured in total pixels as described in Experimental Procedures. Asterisk denotes statistically significant difference against respective control cells, P<0.01 (C) BiFC and αIIbβ3 fluorescence co-localization in transduced cells was evaluated by deconvolution microscopy as described in Experimental Procedures. Data represent 30–60 cells analyzed for each treatment. (D) Western blots were performed to monitor expression of talin and kindlin-2 in cell lysates. The cell lysates were from both uninfected and virus transduced cells whereas only virus transduced cells were analyzed in (A), (B) and (C).
Figure 6. Effect of talin or THD on interaction between kindlins and αIIbβ3.
(A) Schematic illustration of BiFC in CHO cells depicting αIIb-VC, VN-kindlin-2 and β3. When VN-kindlin interacts with αIIb-VCβ3 through the β3 tail, VN and VC should reconstitute Venus, resulting in BiFC. (B) Over-expression of THD or talin does not promote kindlin-2 interaction with αIIbβ3. CHO cells expressing αIIb-VCβ3 or αIIb-VCβ3Δ724 was co-transfected with Tac as a transfection marker and an expression vector for THD, talin or empty vector (Mock), as indicated. After induction of VN-kindlin-2 expression with doxycycline, BiFC was quantified by flow cytometry. BiFC fluorescence was normalized to αIIbβ3 expression and expressed as fold-increase relative to doxycycline-treated, Mock-transfected cells. Data represent means ± SEM of three experiments (asterisk denotes statistically significant difference against mock/induced, P<0.05). (C) Western blots were performed to monitor expression of VN-kindlin-2, talin and THD in cell lysates. (D) Purified kindlin-3 with or without addition of THD was incubated with the recombinant β3 cytoplasmic tail conjugated to neutravidin beads. After washing, proteins bound to the beads were detected on western blots. Band intensities were quantified in LICOR, normalized to kindlin-3 binding in the absence of THD, and presented as a curve. Insert shows a representative western blot of 3 independent experiments. Increasing amount of β3 tail bound THD failed to promote β3–kindlin-3 interaction. Data represent means ± SEM of three experiments. (E) Similar to (D) but talin was used instead of THD. Increasing amounts of β3 tail-bound talin failed to promote β3–kindlin-3 interaction. (asterisks in D and E denotes statistically significant differences compared to kindlin binding in the absence of talin or THD, P<0.10).
Similar articles
- Kindlin-1 and -2 directly bind the C-terminal region of beta integrin cytoplasmic tails and exert integrin-specific activation effects.
Harburger DS, Bouaouina M, Calderwood DA. Harburger DS, et al. J Biol Chem. 2009 Apr 24;284(17):11485-97. doi: 10.1074/jbc.M809233200. Epub 2009 Feb 23. J Biol Chem. 2009. PMID: 19240021 Free PMC article. - A conserved lipid-binding loop in the kindlin FERM F1 domain is required for kindlin-mediated αIIbβ3 integrin coactivation.
Bouaouina M, Goult BT, Huet-Calderwood C, Bate N, Brahme NN, Barsukov IL, Critchley DR, Calderwood DA. Bouaouina M, et al. J Biol Chem. 2012 Mar 2;287(10):6979-90. doi: 10.1074/jbc.M111.330845. Epub 2012 Jan 10. J Biol Chem. 2012. PMID: 22235127 Free PMC article. - ADAP interactions with talin and kindlin promote platelet integrin αIIbβ3 activation and stable fibrinogen binding.
Kasirer-Friede A, Kang J, Kahner B, Ye F, Ginsberg MH, Shattil SJ. Kasirer-Friede A, et al. Blood. 2014 May 15;123(20):3156-65. doi: 10.1182/blood-2013-08-520627. Epub 2014 Feb 12. Blood. 2014. PMID: 24523237 Free PMC article. - Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart.
Chen C, Manso AM, Ross RS. Chen C, et al. Pediatr Cardiol. 2019 Oct;40(7):1401-1409. doi: 10.1007/s00246-019-02167-3. Epub 2019 Jul 31. Pediatr Cardiol. 2019. PMID: 31367953 Free PMC article. Review. - Kindlin: helper, co-activator, or booster of talin in integrin activation?
Ye F, Petrich BG. Ye F, et al. Curr Opin Hematol. 2011 Sep;18(5):356-60. doi: 10.1097/MOH.0b013e3283497f09. Curr Opin Hematol. 2011. PMID: 21730832 Review.
Cited by
- Talin and kindlin use integrin tail allostery and direct binding to activate integrins.
Aretz J, Aziz M, Strohmeyer N, Sattler M, Fässler R. Aretz J, et al. Nat Struct Mol Biol. 2023 Dec;30(12):1913-1924. doi: 10.1038/s41594-023-01139-9. Epub 2023 Dec 12. Nat Struct Mol Biol. 2023. PMID: 38087085 Free PMC article. - Structural Basis of β2 Integrin Inside-Out Activation.
Wen L, Lyu Q, Ley K, Goult BT. Wen L, et al. Cells. 2022 Sep 28;11(19):3039. doi: 10.3390/cells11193039. Cells. 2022. PMID: 36231001 Free PMC article. Review. - Mechanism of integrin activation by talin and its cooperation with kindlin.
Lu F, Zhu L, Bromberger T, Yang J, Yang Q, Liu J, Plow EF, Moser M, Qin J. Lu F, et al. Nat Commun. 2022 Apr 29;13(1):2362. doi: 10.1038/s41467-022-30117-w. Nat Commun. 2022. PMID: 35488005 Free PMC article. - Talin‑1 interaction network in cellular mechanotransduction (Review).
Zhao Y, Lykov N, Tzeng C. Zhao Y, et al. Int J Mol Med. 2022 May;49(5):60. doi: 10.3892/ijmm.2022.5116. Epub 2022 Mar 10. Int J Mol Med. 2022. PMID: 35266014 Free PMC article. Review. - Molecular mechanisms of leukocyte β2 integrin activation.
Wen L, Moser M, Ley K. Wen L, et al. Blood. 2022 Jun 16;139(24):3480-3492. doi: 10.1182/blood.2021013500. Blood. 2022. PMID: 35167661 Free PMC article. Review.
References
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. - PubMed
- Bertagnolli ME, Locke SJ, Hensler ME, Bray PF, Beckerle MC. Talin distribution and phosphorylation in thrombin-activated platelets. J Cell Sci. 1993;106(Pt 4):1189–1199. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 GM094663/GM/NIGMS NIH HHS/United States
- T32 HL086344/HL/NHLBI NIH HHS/United States
- GM094663/GM/NIGMS NIH HHS/United States
- GM098412/GM/NIGMS NIH HHS/United States
- R01 HL056595/HL/NHLBI NIH HHS/United States
- HL56595/HL/NHLBI NIH HHS/United States
- P01 GM098412/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources