Modeling vaccination campaigns and the Fall/Winter 2009 activity of the new A(H1N1) influenza in the Northern Hemisphere - PubMed (original) (raw)
doi: 10.3134/ehtj.09.011. Epub 2009 Nov 2.
C Poletto, D Balcan, H Hu, B Goncalves, Jj Ramasco, D Paolotti, N Perra, M Tizzoni, W Van den Broeck, V Colizza, A Vespignani
Affiliations
- PMID: 22460281
- PMCID: PMC3167647
- DOI: 10.3134/ehtj.09.011
Modeling vaccination campaigns and the Fall/Winter 2009 activity of the new A(H1N1) influenza in the Northern Hemisphere
P Bajardi et al. Emerg Health Threats J. 2009.
Abstract
The unfolding of pandemic influenza A(H1N1) for Fall 2009 in the Northern Hemisphere is still uncertain. Plans for vaccination campaigns and vaccine trials are underway, with the first batches expected to be available early October. Several studies point to the possibility of an anticipated pandemic peak that could undermine the effectiveness of vaccination strategies. Here, we use a structured global epidemic and mobility metapopulation model to assess the effectiveness of massive vaccination campaigns for the Fall/Winter 2009. Mitigation effects are explored depending on the interplay between the predicted pandemic evolution and the expected delivery of vaccines. The model is calibrated using recent estimates on the transmissibility of the new A(H1N1) influenza. Results show that if additional intervention strategies were not used to delay the time of pandemic peak, vaccination may not be able to considerably reduce the cumulative number of cases, even when the mass vaccination campaign is started as early as mid-October. Prioritized vaccination would be crucial in slowing down the pandemic evolution and reducing its burden.
Conflict of interest statement
Competing interests: The authors have declared that no competing interests exist.
Figures
Figure 1
Compartmental structure in each sub-population. A susceptible individual interacting with an infectious person may contract the illness and enter the latent compartment where he is infected, but not yet infectious. At the end of the latency period, each latent individual becomes infectious entering the symptomatic compartment with probability (1-pa) or becoming asymptomatic with probability pa. Asymptomatic individuals infect with a transmission rate reduced of rβ. A fraction (1-pt) of the symptomatic individuals would stop traveling when ill. Infectious individuals recover permanently with rate µ. Antiviral treatment is assumed to be administered to a fraction pAV of the symptomatic infectious individuals within 1 day from the onset of symptoms, according to the drugs availability in the country. It reduces the infectiousness by the antiviral efficacy AVEI and shortens the infectious period of 1 day. If vaccines are available, a fraction equal to 1% of the susceptible population enters the susceptible vaccinated compartment each day. A similar progression to the baseline compartmentalization is considered if infection occurs. However, the vaccine reduces the susceptibility of the vaccinated susceptible with an efficacy VEs, the probability of developing symptoms if infection occurs with an efficacy VED, and their transmission rate while infectious with an efficacy VEi. All transition process are modeled through multinomial processes.
Figure 2
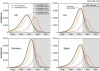
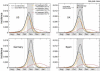
Effect of vaccination and of combined strategies for the early peak case. The incidence curves show the impact of an incremental vaccination with 1% daily distribution policy starting on 15 October for the early peak case. The baseline case is compared with the cases in which intervention strategies are considered, vaccination only, and combination of vaccination with antiviral treatment of 5, 10 and 30% of clinical cases. Efficacies of antiviral treatment and vaccination assume the values reported in the main text. Median profiles obtained from 2000 stochastic realizations of the model are shown. A 60% vaccine coverage is assumed, with the gray bar indicating the time period during which the immunization takes effect.
Figure 3
Effect of vaccination and of combined strategies for the late peak case. The incidence curves show the impact of an incremental vaccination with 1% daily distribution policy starting on 15 October for the late peak case. The baseline case is compared with the cases in which intervention strategies are considered, vaccination only, and combination of vaccination with antiviral treatment of 5, 10 and 30% of clinical cases. Efficacies of antiviral treatment and vaccination assume the values reported in the main text. Median profiles obtained from 2000 stochastic realizations of the model are shown. A 30% coverage is assumed, with the gray bar indicating the time period during which the immunization takes effect.
Similar articles
- Seasonal transmission potential and activity peaks of the new influenza A(H1N1): a Monte Carlo likelihood analysis based on human mobility.
Balcan D, Hu H, Goncalves B, Bajardi P, Poletto C, Ramasco JJ, Paolotti D, Perra N, Tizzoni M, Van den Broeck W, Colizza V, Vespignani A. Balcan D, et al. BMC Med. 2009 Sep 10;7:45. doi: 10.1186/1741-7015-7-45. BMC Med. 2009. PMID: 19744314 Free PMC article. - On Temporal Patterns and Circulation of Influenza Virus Strains in Taiwan, 2008-2014: Implications of 2009 pH1N1 Pandemic.
Hsieh YH, Huang HM, Lan YC. Hsieh YH, et al. PLoS One. 2016 May 3;11(5):e0154695. doi: 10.1371/journal.pone.0154695. eCollection 2016. PLoS One. 2016. PMID: 27139905 Free PMC article. - Vaccines against influenza A (H1N1) pandemic.
Valdespino-Gomez JL, Garcia-Garcia L, de León-Rosales SP. Valdespino-Gomez JL, et al. Arch Med Res. 2009 Nov;40(8):693-704. doi: 10.1016/j.arcmed.2009.10.008. Arch Med Res. 2009. PMID: 20304259 - Vaccination against influenza: role and limitations in pandemic intervention plans.
Rebmann T, Zelicoff A. Rebmann T, et al. Expert Rev Vaccines. 2012 Aug;11(8):1009-19. doi: 10.1586/erv.12.63. Expert Rev Vaccines. 2012. PMID: 23002981 Review. - Factors influencing pandemic influenza vaccination of healthcare workers--a systematic review.
Prematunge C, Corace K, McCarthy A, Nair RC, Pugsley R, Garber G. Prematunge C, et al. Vaccine. 2012 Jul 6;30(32):4733-43. doi: 10.1016/j.vaccine.2012.05.018. Epub 2012 May 27. Vaccine. 2012. PMID: 22643216 Review.
Cited by
- Human mobility and the worldwide impact of intentional localized highly pathogenic virus release.
Gonçalves B, Balcan D, Vespignani A. Gonçalves B, et al. Sci Rep. 2013;3:810. doi: 10.1038/srep00810. Sci Rep. 2013. PMID: 23860371 Free PMC article. - Predicting and controlling infectious disease epidemics using temporal networks.
Masuda N, Holme P. Masuda N, et al. F1000Prime Rep. 2013;5:6. doi: 10.12703/P5-6. Epub 2013 Mar 4. F1000Prime Rep. 2013. PMID: 23513178 Free PMC article. - Towards a characterization of behavior-disease models.
Perra N, Balcan D, Gonçalves B, Vespignani A. Perra N, et al. PLoS One. 2011;6(8):e23084. doi: 10.1371/journal.pone.0023084. Epub 2011 Aug 3. PLoS One. 2011. PMID: 21826228 Free PMC article. - The GLEaMviz computational tool, a publicly available software to explore realistic epidemic spreading scenarios at the global scale.
Van den Broeck W, Gioannini C, Gonçalves B, Quaggiotto M, Colizza V, Vespignani A. Van den Broeck W, et al. BMC Infect Dis. 2011 Feb 2;11:37. doi: 10.1186/1471-2334-11-37. BMC Infect Dis. 2011. PMID: 21288355 Free PMC article. Review. - Real-time numerical forecast of global epidemic spreading: case study of 2009 A/H1N1pdm.
Tizzoni M, Bajardi P, Poletto C, Ramasco JJ, Balcan D, Gonçalves B, Perra N, Colizza V, Vespignani A. Tizzoni M, et al. BMC Med. 2012 Dec 13;10:165. doi: 10.1186/1741-7015-10-165. BMC Med. 2012. PMID: 23237460 Free PMC article.
References
- H1N1 Flu Situation Update. Centers for Disease Control and Prevention. 2009. http://www.cdc.gov/h1n1flu/update.htm.
- World Health Organization, Pandemic (H1N1) 2009. update 66 (September 18, 2009) http://www.who.int/csr/don/2009_09_18/en/index.html.
- BBC news, fears swine flu ‘on the way back’. http://news.bbc.co.uk/2/hi/health/8261496.stm.
- Report to the President on US preparations for 2009-H1N1 influenza, Executive Office of the President, President's Council of Advisors on Science and Technology; 2009.
- European Centre for Disease Prevention and Control, New ECDC pandemic risk assessment. http://www.ecdc.europa.eu/en/press/news/Lists/News/ECDC_DispForm.aspx?Li....
LinkOut - more resources
Full Text Sources