Dual functions of the Hsm3 protein in chaperoning and scaffolding regulatory particle subunits during the proteasome assembly - PubMed (original) (raw)
. 2012 Apr 24;109(17):E1001-10.
doi: 10.1073/pnas.1116538109. Epub 2012 Mar 29.
Nicolas Richet, Chloe Godard, Brice Murciano, Benoît Le Tallec, Erwann Rousseau, Pierre Legrand, Jean-Baptiste Charbonnier, Marie-Hélène Le Du, Raphaël Guérois, Françoise Ochsenbein, Anne Peyroche
Affiliations
- PMID: 22460800
- PMCID: PMC3340050
- DOI: 10.1073/pnas.1116538109
Dual functions of the Hsm3 protein in chaperoning and scaffolding regulatory particle subunits during the proteasome assembly
Marie-Bénédicte Barrault et al. Proc Natl Acad Sci U S A. 2012.
Abstract
The 26S proteasome, a molecular machine responsible for regulated protein degradation, consists of a proteolytic core particle (20S CP) associated with 19S regulatory particles (19S RPs) subdivided into base and lid subcomplexes. The assembly of 19S RP base subcomplex is mediated by multiple dedicated chaperones. Among these, Hsm3 is important for normal growth and directly targets the carboxyl-terminal (C-terminal) domain of Rpt1 of the Rpt1-Rpt2-Rpn1 assembly intermediate. Here, we report crystal structures of the yeast Hsm3 chaperone free and bound to the C-terminal domain of Rpt1. Unexpectedly, the structure of the complex suggests that within the Hsm3-Rpt1-Rpt2 module, Hsm3 also contacts Rpt2. We show that in both yeast and mammals, Hsm3 actually directly binds the AAA domain of Rpt2. The Hsm3 C-terminal region involved in this interaction is required in vivo for base assembly, although it is dispensable for binding Rpt1. Although Rpt1 and Rpt2 exhibit weak affinity for each other, Hsm3 unexpectedly acts as an essential matchmaker for the Rpt1-Rpt2-Rpn1 assembly by bridging both Rpt1 and Rpt2. In addition, we provide structural and biochemical evidence on how Hsm3/S5b may regulate the 19S RP association to the 20S CP proteasome. Our data point out the diverse functions of assembly chaperones.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
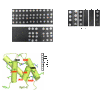
Hsm3 structure is a curve-shaped croissant composed of 11 Arm/HEAT repeats. (A) Cartoon representation of the crystal structure of Hsm3 in two orthogonal views. The ribbon is colored with a rainbow gradient from blue to red from the N terminus to the C terminus, respectively. Each Arm/HEAT repeat is labeled from R1 (repeat 1) to R11 (repeat 11). (B) Surface representation of the model colored with respect to the conservation grade of the sequences calculated with the Rate4Site algorithm (53). The Rate4Site evolutionary rates were binned into 10 intervals and were assigned a color ranging from white (most variable) to red (most conserved), with intermediate shades of yellow and orange. The structure is presented in two orthogonal views as in A.
Fig. 2.
Cter domain of Rpt1 binds to the center of the concave face of Hsm3. (A) Cartoon representation of the crystal structure of Hsm3 (in orange) bound to the Cter domain of yeast Rpt1, Rpt1-C (in green), with the four helices of Rpt1-C labeled (α1–α4). (B) Close-up view of residues involved in the specific recognition of Rpt1-C (in green) by Hsm3 (in orange) and corresponding to the boxed region in A. Hydrophobic residues in close contact are represented as spheres, whereas polar and charged residues are shown as sticks. Polar contacts are shown with magenta dashed lines. Helices of Hsm3 and Rpt1 involved in the binding interface are indicated in the figure. The side chains of residues in contact (<5 Å) are shown and labeled outside the box. Mutated residues are highlighted with the following code. Residues for which interaction is severely or moderately affected on mutation as reported by the two-hybrid assay are boxed by a continuous or dashed line, respectively (Fig. 3). Residues mutated without perturbing the interaction are underlined. Asterisks highlight two residues, D230 in Hsm3 and R403 in Rpt1, for which a charge compensatory effect was detected on charge reversal mutations (Fig. 3_C_). (C) Cartoon representation with solid cylinder helices and transparent representation of the surface of Hsm3 (orange) bound to the complete ATPase domain of Rpt1 (green). The ATPase domain is divided into two subdomains: the Nter domain shown in light green labeled Rpt1AAA and the Cter domain shown in darker green labeled Rpt1-C. The position of the Rpt1AAA domain has been modeled as described in Materials and Methods. The position of the Rpt1-C domain was observed in the crystal structure of the Hsm3–Rpt1-C complex. (D) Model of Hsm3 in complex with the ATPase domains of Rpt1 and Rpt2. Hsm3 is represented with an orange surface. Hsm3 C-terminal residues deleted in Hsm3ΔC (labeled Hsm3Cter) are shown in light orange (main text and Figs. 3_A_ and 6). The Rpt1 ATPase domain is depicted as in C. The position of Rpt2 (in blue) has been modeled as described in Materials and Methods. In A_–_D, the orientation of Hsm3 is identical to that presented in Fig. 1 (Upper), and an additional view after 90° of rotation is added in D.
Fig. 3.
Hsm3-Rpt1–specific binding involves a hydrophobic patch surrounded by polar interactions. (A) Various constructs of Hsm3 [full-length (FL), Hsm3 truncated for the first 115 residues (ΔN), or Hsm3 truncated for the last 104 residues (ΔC)] in fusion with the Gal4-DNA binding domain or the corresponding pGBT9 empty vector (−) were introduced into cells with full-length Rpt1 (Rpt1FL), the last 90 residues of Rpt1 (Rpt1-C) in fusion with Gal4-AD, or the corresponding pACT2 empty vector (−). Serial dilutions of diploids were tested for growth onto control plates [synthetic dextrose (SD)] and for transcriptional activation of the HIS3 reporter gene onto plates containing 3AT. (B) Cartoon representation of Rpt1-C indicating some of the potential interacting residues shown as sticks. Residues tested by mutagenesis are indicated. The coloring code is as follows: the interaction with Hsm3 is abolished in mutants corresponding to red residues, whereas the binding is efficient for mutants corresponding to black residues. The asterisk indicates different results depending on the nature of the substitution. (Right) Table recapitulates the mutations tested. The signs “+++,” “++,” and “−” correspond to very strong, strong, and weak interaction with Hsm3 in the two-hybrid assay, respectively. (C) Three different versions of full-length Hsm3 (WT, D230A, or D230R) fused to Gal4-AD in the pACT2 vector were introduced in Y190. The transformants were mated with Y187 strain containing Gal4-DNA binding domain–Rpt1-C (WT or R403E). The growth of diploid cells was tested in the presence of 3AT to evaluate the HIS3 reporter activation.
Fig. 4.
Hsm3 directly binds Rpn1. (A) Hsm3 was fused to the Gal4-DNA binding domain (Gal4-DBD), and Rpn1 was fused to the Gal4-AD. Empty vectors (−) (pGBT9 and pACT2, respectively) were used as negative controls. Serial dilutions of diploids containing the various combinations of Gal4 fusions were plated in the presence of various concentrations of 3AT as indicated to evaluate transcriptional activation of the HIS3. SD, synthetic dextrose. (B) Individually expressed (lanes 1, 2, and 5) or coexpressed (lanes 3 and 6) of recombinant His-GST-Hsm3 (GST-Hsm3) or Rpt1[377–467]-maltose binding protein (Rpt1-MBP) proteins were produced in E. coli cells. Soluble extracts were loaded onto glutathione agarose. Soluble extracts of E. coli cells expressing Rpn1 (lanes 4, 5, and 6) or not (lanes 1, 2, and 3) were then added. After washing, bound proteins were eluted by adding glutathione disulfide (GSH) and analyzed by Coomassie staining. Extracts from _E. coli_-containing corresponding empty vectors were used as controls.
Fig. 5.
Rpn1 binds the Nter domain of Rpt2, whereas Hsm3 binds the Rpt2AAA domain. (A) Full-length Rpt2 (FL), Rpt2 truncated for the last 270 residues (ΔAAA), or Rpt2 truncated for the first 50 residues (ΔNter) was fused to the Gal4-DNA binding domain in pASΔ vector. Rpn1 was fused to Gal4-AD in pACT2 vector. Diploids containing various combinations of both plasmids were plated onto selective plates in the presence of 3AT to monitor the transcriptional activation of HIS3 gene reporter. pASΔ empty vector (−) was used as a control. SD, synthetic dextrose. (B) Interaction of recombinant Rpn1 and Rpt2. (Left) Soluble extracts of E. coli expressing Rpt2 individually (lane 1), expressing Rpt2 individually and mixed with extracts expressing His6-Rpn1 just before cell lysis (lane 2), or coexpressing Rpt2 with His6-Rpn1 using the pRSFDuet vector (lane 3) were prepared. The solubility of Rpt2 was monitored by Western blotting using anti-Rpt2 antibodies. Stars indicate nonrelevant cross-reacting proteins. (Right) Soluble extracts from E. coli cells (corresponding to lanes 1, 2, and 3 as described above) were loaded onto Ni++-NTA agarose. As controls, soluble extracts from cells expressing no recombinant protein (C1) or expressing His6-Rpn1 only (C2) were added. After washing, bound proteins were eluted by adding imidazole to a final concentration of 250 mM. Recombinant His6-Rpn1 and Rpt2 were detected by Western blotting using anti-His or anti-Rpt2 antibodies, respectively. The dashed line indicates degradation products of recombinant His6-Rpn1. The star indicates nonrelevant cross-reacting proteins. (C) Schematic cartoon of the different structural domains of Rpt2. Various constructs of the Rpt2 subunit, depicted as dark lines, were fused to a bait plasmid and tested as described in A for interaction with Hsm3, Rpn1, Rpt6, and Rpt1 fused to a prey plasmid. The signs “+,” “+/−,” and “−“” correspond to specifically significant, weak, and null growth in the presence of 3AT, respectively. (D) Yeast cells containing pACT2-Hsm3 (Hsm3) or the corresponding empty vector (vector) and pGBT9-Rpt1, pASΔ-Rpt2, or corresponding empty vectors (−) were spotted onto control plates [synthetic dextrose (SD)] and onto plates containing 3AT to monitor activation of HIS reporter. (E) As in D, except that the bait corresponds to S5b fused to Gal4-AD and we added human Rpt2 as prey (hRpt2). yRpt1 and yRpt2 correspond to yeast Rpt1 and yeast Rpt2 fused to the Gal4-DNA binding domain as described in D.
Fig. 6.
Cter domain of Hsm3 is specifically required for interacting with Rpt2 and is essential in vivo to assemble the Rpn1–Rpt2–Rpt1–Hsm3 complex. (A) Full-length Hsm3 (FL) or Hsm3 truncated for the last 104 residues (ΔC) in fusion with the Gal4-DNA binding domain or the corresponding pGBT9 empty vector (−) was combined with full-length Rpt2 in fusion with Gal4-AD. Resulting diploids were tested for growth onto control plates [synthetic dextrose (SD)] and for transcriptional activation of the HIS3 reporter gene onto plates containing 3AT. (B) Recombinant full-length His-GST-Hsm3 (FL) or truncated His-GST-Hsm3ΔC (ΔC) proteins individually expressed (lanes 2 and 4) or coexpressed with Rpt1-maltose binding protein (Rpt1FL; lanes 3, 5, 6, and 7) were produced in E. coli cells. E. coli cells expressing Rpn1 (lanes 6 and 7) or not (lanes 1, 2, 3, 4, and 5) were added before lysis. Resulting soluble extracts were loaded onto glutathione agarose. After washing, bound proteins were eluted by adding glutathione disulfide and analyzed by Simply Safe blue staining. Extracts from E. coli containing corresponding empty vectors were used as controls. (C) Tenfold serial dilutions of WT, _hsm3_Δ (Δ), or hsm3_Δ_C (ΔC) strains were spotted onto YPD plates and incubated at the indicated temperatures for 2 d. (D) Tetrads resulting from the sporulation of heterozygous double mutants (_RPN4/rpn4_Δ HSM3/hsm3_Δ_C) were incubated at 30 °C onto YPD plates. The relevant genotype of each strain is indicated. (E) Different strains modified in RPN4 locus and/or HSM3 locus, as indicated, were tested for growth. Fivefold serial dilutions were spotted onto YPD plates and incubated at the indicated temperatures for 2 d. (F) Protein extracts from yeast strains grown at 30 °C or 37 °C and expressing full-length Hsm3-Myc (FL) or the C-terminal truncated version of Hsm3-Myc (ΔC) were immunoprecipitated with anti-Myc antibodies. Total extracts (Input) and immunoprecipitated proteins (IP Myc) were analyzed by Western blotting using the antibodies indicated on the left. (G) Cells expressing WT full-length Hsm3 (WT), Hsm3ΔC (ΔC), full-length Myc-tagged Hsm3 (Myc), or C-terminal truncated Myc-tagged Hsm3 (ΔC Myc) were grown in minimal medium at 37 °C. Protein extracts were resolved by native PAGE. Hsm3-, Rpt1-, Rpt2-, and Rpn1-containing species were detected by Western blot analyses using the indicated antibodies. Black arrowheads indicate Rpn1–Rpt2–Rpt1–Hsm3 complex, and white arrowheads indicate Rpn1–Rpt2–Rpt1–Hsm3Myc complex that migrates differently because of the presence of the 13-Myc tag. The asterisk indicates a nonspecific, cross-reacting band.
Fig. 7.
Hsm3 is a matchmaker for Rpt1–Rpt2–Rpn1 assembly module. (A) Mutations in Rpt1 abrogating Hsm3 binding combined with a noncompetent form of Hsm3 for Rpt2 binding phenocopy loss of Hsm3 function. Fivefold serial dilutions of WT, hsm3_Δ (Δ), or hsm3_Δ_C (ΔC) strains containing WT Rpt1 (WT) or a mutant version of Rpt1 (E339R, R403E, R409E, L406E, and L410R) were spotted onto YPD plates and incubated at the indicated temperatures for 2 d. (B) Summary of the interactions within the Hsm3 base assembly module detected in this study and by Le Tallec et al. (19). The different domains of Rpt1 and Rpt2 are represented in accordance with what is described in Fig. 5_C. Arrows correspond to interactions detected in the two-hybrid system, and dotted lines are used to direct interactions between recombinant proteins. The thickness represents the relative strength of interactions. (C) Unique functions of Hsm3 in Hsm3 module assembly. The Rpn1–Rpt2 module preexists and is supported by the binding of Rpn1 to the CC domain of Rpt2. (Left) Hsm3–Rpt1 module associates with Rpn1-Rpt2 notably, thanks to the Hsm3-Rpt2 interaction involving the Cter domain of Hsm3 and the AAA domain of Rpt2. (Right) Impairing this interaction precludes the formation of the entire module.
Fig. 8.
Hsm3 is part of base-like and 19S RP-like complexes but precludes binding to 20S CP. (A) Protein extracts from WT cells or cells overexpressing WT full-length Hsm3 (2μ HSM3+) or not (2μ HSM3−) were resolved by native PAGE. Hsm3-containing species were detected by Western blot analyses using specific antibodies. At least three distinct Hsm3-containing complexes are detected: Rpn1–Rpt2–Rpt1–Hsm3 complex (I), a base-like complex (II), and a 19S RP-like complex (III). When overexpressed, Hsm3 also accumulates in its free form as indicated. (B) Overexpression of Hsm3 impairs the association between 20S CP and 19S RP. Rpn1-TAP or Rpn9-TAP strain was transformed with a plasmid overexpressing HSM3 (2μ HSM3+) or with the empty vector (2μ _HSM3_−). IgG immunoprecipitations were then performed. Coimmunoprecipitated proteins were evaluated by Western blotting with appropriate antibodies, as indicated on the right. (C) Model of the AAA ATPase Rpt ring from the EM structure of the yeast 26S proteasome in complex with Hsm3 obtained as described in
SI Materials and Methods
. Proteins are shown as cartoons with solid cylinder helices. Rpt1 helices are shown in green, Rpt2 is shown in blue, and Hsm3 is shown in orange. The other AAA ATPase domains are shown in cyan. The surface of the AAA ATPase ring and that of the 20S CP are shown with 50% transparency. (Right) Position of the 20S CP is shown in gray.
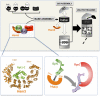
Fig. P1.
(Upper) Eukaryotic 26S proteasome, responsible for protein degradation, consists of a base, lid, and 20S CP. Base assembly is mediated by four dedicated assembly chaperones, including Hsm3, in yeast. They participate in different assembly intermediates, but the exact sequence of assembly events is still unclear. Each chaperone interacts with the carboxyl-terminal domain of a specific Rpt subunit, as indicated. Hsm3 is the only chaperone that is part of a precursor complex containing two ATPase subunits (Rpt1 and Rpt2) plus a non-ATPase subunit (Rpn1). (Lower Left) Cartoon representation of the crystal structure of Hsm3 (in orange) bound to the C-terminal domain of yeast Rpt1 (Rpt1-C) (in green). (Lower Right) Unique features of the Hsm3 chaperone. The Hsm3–Rpt1 module associates with the preexisting Rpn1-Rpt2, notably thanks to the Hsm3–Rpt2 interaction involving the C-terminal domain of Hsm3. Impairing this interaction precludes the formation of the entire module. Nter, N-terminus; Cter, C-terminus.
Similar articles
- Structural basis for specific recognition of Rpt1p, an ATPase subunit of 26 S proteasome, by proteasome-dedicated chaperone Hsm3p.
Takagi K, Kim S, Yukii H, Ueno M, Morishita R, Endo Y, Kato K, Tanaka K, Saeki Y, Mizushima T. Takagi K, et al. J Biol Chem. 2012 Apr 6;287(15):12172-82. doi: 10.1074/jbc.M112.345876. Epub 2012 Feb 8. J Biol Chem. 2012. PMID: 22334676 Free PMC article. - Hsm3/S5b participates in the assembly pathway of the 19S regulatory particle of the proteasome.
Le Tallec B, Barrault MB, Guérois R, Carré T, Peyroche A. Le Tallec B, et al. Mol Cell. 2009 Feb 13;33(3):389-99. doi: 10.1016/j.molcel.2009.01.010. Mol Cell. 2009. PMID: 19217412 - Multiple assembly chaperones govern biogenesis of the proteasome regulatory particle base.
Funakoshi M, Tomko RJ Jr, Kobayashi H, Hochstrasser M. Funakoshi M, et al. Cell. 2009 May 29;137(5):887-99. doi: 10.1016/j.cell.2009.04.061. Epub 2009 May 14. Cell. 2009. PMID: 19446322 Free PMC article. - Assembly and function of the proteasome.
Saeki Y, Tanaka K. Saeki Y, et al. Methods Mol Biol. 2012;832:315-37. doi: 10.1007/978-1-61779-474-2_22. Methods Mol Biol. 2012. PMID: 22350895 Review. - Assembly manual for the proteasome regulatory particle: the first draft.
Park S, Tian G, Roelofs J, Finley D. Park S, et al. Biochem Soc Trans. 2010 Feb;38(Pt 1):6-13. doi: 10.1042/BST0380006. Biochem Soc Trans. 2010. PMID: 20074027 Free PMC article. Review.
Cited by
- Nuclear Import of Yeast Proteasomes.
Burcoglu J, Zhao L, Enenkel C. Burcoglu J, et al. Cells. 2015 Aug 7;4(3):387-405. doi: 10.3390/cells4030387. Cells. 2015. PMID: 26262643 Free PMC article. Review. - An Allosteric Interaction Network Promotes Conformation State-Dependent Eviction of the Nas6 Assembly Chaperone from Nascent 26S Proteasomes.
Nemec AA, Peterson AK, Warnock JL, Reed RG, Tomko RJ Jr. Nemec AA, et al. Cell Rep. 2019 Jan 8;26(2):483-495.e5. doi: 10.1016/j.celrep.2018.12.042. Cell Rep. 2019. PMID: 30625330 Free PMC article. - 1.15 Å resolution structure of the proteasome-assembly chaperone Nas2 PDZ domain.
Singh CR, Lovell S, Mehzabeen N, Chowdhury WQ, Geanes ES, Battaile KP, Roelofs J. Singh CR, et al. Acta Crystallogr F Struct Biol Commun. 2014 Apr;70(Pt 4):418-23. doi: 10.1107/S2053230X14003884. Epub 2014 Mar 25. Acta Crystallogr F Struct Biol Commun. 2014. PMID: 24699731 Free PMC article. - The ubiquitin-proteasome system of Saccharomyces cerevisiae.
Finley D, Ulrich HD, Sommer T, Kaiser P. Finley D, et al. Genetics. 2012 Oct;192(2):319-60. doi: 10.1534/genetics.112.140467. Genetics. 2012. PMID: 23028185 Free PMC article. Review. - Two alternative mechanisms regulate the onset of chaperone-mediated assembly of the proteasomal ATPases.
Nahar A, Fu X, Polovin G, Orth JD, Park S. Nahar A, et al. J Biol Chem. 2019 Apr 19;294(16):6562-6577. doi: 10.1074/jbc.RA118.006298. Epub 2019 Feb 27. J Biol Chem. 2019. PMID: 30814255 Free PMC article.
References
- Voges D, Zwickl P, Baumeister W. The 26S proteasome: A molecular machine designed for controlled proteolysis. Annu Rev Biochem. 1999;68:1015–1068. - PubMed
- Goldberg AL. Functions of the proteasome: From protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans. 2007;35(Pt 1):12–17. - PubMed
- Wolf DH, Hilt W. The proteasome: A proteolytic nanomachine of cell regulation and waste disposal. Biochim Biophys Acta. 2004;1695(1–3):19–31. - PubMed
- Groll M, et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature. 1997;386:463–471. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous