Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape - PubMed (original) (raw)
Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape
Janis M Taube et al. Sci Transl Med. 2012.
Abstract
Although many human cancers such as melanoma express tumor antigens recognized by T cells, host immune responses often fail to control tumor growth for as yet unexplained reasons. Here, we found a strong association between melanocyte expression of B7-H1 (PD-L1), an immune-inhibitory molecule, and the presence of tumor-infiltrating lymphocytes (TILs) in human melanocytic lesions: 98% of B7-H1(+) tumors were associated with TILs compared with only 28% of B7-H1(-) tumors. Indeed, B7-H1(+) melanocytes were almost always localized immediately adjacent to TILs. B7-H1/TIL colocalization was identified not only in melanomas but also in inflamed benign nevi, indicating that B7-H1 expression may represent a host response to tissue inflammation. Interferon-γ, a primary inducer of B7-H1 expression, was detected at the interface of B7-H1(+) tumors and TILs, whereas none was found in B7-H1(-) tumors. Therefore, TILs may actually trigger their own inhibition by secreting cytokines that drive tumor B7-H1 expression. Consistent with this hypothesis, overall survival of patients with B7-H1(+) metastatic melanoma was significantly prolonged compared with that of patients with B7-H1(-) metastatic melanoma. Therefore, induction of the B7-H1/PD-1 pathway may represent an adaptive immune resistance mechanism exerted by tumor cells in response to endogenous antitumor activity and may explain how melanomas escape immune destruction despite endogenous antitumor immune responses. These observations suggest that therapies that block this pathway may benefit patients with B7-H1(+) tumors.
Figures
Fig. 1
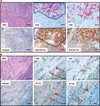
Patterns of B7-H1 expression observed in melanocytic lesions stained with the anti–B7-H1 mAb 5H1. (A) No B7-H1 expression (brown chromogen) by melanocytes in a benign nevus. Original magnification, ×200 (scale bar, 50 µm). Note the paucity of TILs in this case. (B) B7-H1 expression by both melanocytes and TILs at the advancing edge of an invasive primary melanoma, nodular histologic subtype. Original magnification, ×40 (scale bar, 200 µm). (C) Original magnification of the boxed area shown in (B), ×400 (scale bar, 20 µm). Overall, 10% of total melanoma cells in this specimen expressed B7-H1 (scoring was performed as described in Materials and Methods). The inflammatory host response to tumor was graded as “moderate” in this case and included B7-H1+ TILs. (D) Diffuse B7-H1 expression by melanocytes in a subcutaneous melanoma metastasis to the scalp, associated with singular TILs. Original magnification, ×200 (scale bar, 50 µm). Representative photomicrographs of CD3 staining for TILs corresponding to each of these examples are shown in fig. S1. The interface pattern with B7-H1 expression by both melanocytes and TILs at the advancing edge pre-dominated in this study.
Fig. 2
Geographic association of B7-H1 expression and TILs. (A) Primary nodular melanoma with associated “severe” grade of lymphocytic infiltration, highlighted by CD8 immunostaining. The dark line demarcates the area of colocalizing T cells and B7-H1+ tumor cells. Overall, 30% of the tumor cells demonstrated cell surface B7-H1 expression. Note that the melanoma cell cytoplasm is heavily pigmented due to abundant melanin (see isotype control). Original magnifications, ×200 for left and middle column panels (scale bars, 50 µm) and ×400 for right column panels (scale bars, 25 µm). (B) Metastatic deposit of melanoma in a lymph node, associated with “mild” grade of lymphocyte infiltration shown by CD3 immunostaining. Original magnifications, ×100 for left column panels (scale bars, 100 µm), ×200 for middle column panels (scale bars, 50 µm), and ×400 for right column panels (scale bars, 25 µm). Arrows highlight the geographic area of concordance of CD3+ TILs with B7-H1 expression by melanoma, and also point to the area shown at higher magnification in the right-hand panels. Five percent of tumor cells demonstrated B7-H1 expression in this case.
Fig. 3
Distribution of intensity of B7-H1 expression by melanocytic cells and immune infiltrates in 150 lesions. (A) Percent B7-H1 expression by melanocytes ≥5% was considered positive. (B) AIS, defined as the intensity of intratumoral inflammation including TILs and histiocytes (graded 0 to 3, see text) multiplied by the percent B7-H1+ inflammatory cells.
Fig. 4
Correlation of overall patient survival with B7-H1 expression in melanomas. (A) There was no significant difference in survival related to melanoma cell B7-H1 expression in 43 patients with primary invasive melanomas (P = 0.33). (B) However, among 56 patients with metastatic disease, those whose metastatic tumors expressed B7-H1 had significantly improved survival compared to the B7-H1− cohort (P = 0.032). Similar results were seen in analyzing the AIS, representing B7-H1 expression in lymphohistiocytic tumor infiltrates, as well as in analyzing the presence of TILs (mild, moderate, and severe lymphocyte infiltrates with associated histiocytes/macrophages). (C and E) No correlation of AIS or TILs was observed with survival in patients with invasive primary melanomas [P = 0.25 (C) and 0.32 (E)]. (D and F) However, there was a significant correlation in patients with metastatic disease [P = 0.014 (D) and 0.017 (F)]. B7-H1+, ≥5% of tumor cells with membranous expression; B7-H1−, <5% membranous tumor cell expression.
Similar articles
- PD-L1 Expression and Immune Escape in Melanoma Resistance to MAPK Inhibitors.
Kakavand H, Rawson RV, Pupo GM, Yang JYH, Menzies AM, Carlino MS, Kefford RF, Howle JR, Saw RPM, Thompson JF, Wilmott JS, Long GV, Scolyer RA, Rizos H. Kakavand H, et al. Clin Cancer Res. 2017 Oct 15;23(20):6054-6061. doi: 10.1158/1078-0432.CCR-16-1688. Epub 2017 Jul 19. Clin Cancer Res. 2017. PMID: 28724663 - Foci of Programmed Cell Death-Ligand 1 (PD-L1)-positive Tumor Areas With Tumor-infiltrating Leukocytes (TILs) Evocative of a PD-1/PD-L1-related Adaptive Immune Resistance are Frequent in Merkel Cell Carcinoma.
Bénigni P, Guénolé M, Bonsang B, Marcorelles P, Schick U, Uguen A. Bénigni P, et al. Appl Immunohistochem Mol Morphol. 2020 Jan;28(1):17-22. doi: 10.1097/PAI.0000000000000792. Appl Immunohistochem Mol Morphol. 2020. PMID: 31343994 - The relationships between PD-L1 expression, CD8+ TILs and clinico-histomorphological parameters in malignant melanomas.
Škuciová V, Drahošová S, Výbohová D, Cígerová V, Adamkov M. Škuciová V, et al. Pathol Res Pract. 2020 Sep;216(9):153071. doi: 10.1016/j.prp.2020.153071. Epub 2020 Jun 20. Pathol Res Pract. 2020. PMID: 32825944 - Tumor-infiltrating lymphocytes: apparently good for melanoma patients. But why?
Cipponi A, Wieers G, van Baren N, Coulie PG. Cipponi A, et al. Cancer Immunol Immunother. 2011 Aug;60(8):1153-60. doi: 10.1007/s00262-011-1026-2. Epub 2011 May 7. Cancer Immunol Immunother. 2011. PMID: 21553145 Free PMC article. Review. - B7-H7: A potential target for cancer immunotherapy.
Su Q, Du J, Xiong X, Xie X, Wang L. Su Q, et al. Int Immunopharmacol. 2023 Aug;121:110403. doi: 10.1016/j.intimp.2023.110403. Epub 2023 Jun 6. Int Immunopharmacol. 2023. PMID: 37290327 Review.
Cited by
- Profiling non-small cell lung cancer reveals that PD-L1 is associated with wild type EGFR and vascular invasion, and immunohistochemistry quantification of PD-L1 correlates weakly with RT-qPCR.
Alwithenani A, Bethune D, Castonguay M, Drucker A, Flowerdew G, Forsythe M, French D, Fris J, Greer W, Henteleff H, MacNeil M, Marignani P, Morzycki W, Plourde M, Snow S, Marcato P, Xu Z. Alwithenani A, et al. PLoS One. 2021 May 6;16(5):e0251080. doi: 10.1371/journal.pone.0251080. eCollection 2021. PLoS One. 2021. PMID: 33956842 Free PMC article. - The Crosstalk between Microbiome and Immune Response in Gastric Cancer.
Nasr R, Shamseddine A, Mukherji D, Nassar F, Temraz S. Nasr R, et al. Int J Mol Sci. 2020 Sep 9;21(18):6586. doi: 10.3390/ijms21186586. Int J Mol Sci. 2020. PMID: 32916853 Free PMC article. Review. - Targeting the Tumor Microenvironment for Improving Therapeutic Effectiveness in Cancer Immunotherapy: Focusing on Immune Checkpoint Inhibitors and Combination Therapies.
Chyuan IT, Chu CL, Hsu PN. Chyuan IT, et al. Cancers (Basel). 2021 Mar 10;13(6):1188. doi: 10.3390/cancers13061188. Cancers (Basel). 2021. PMID: 33801815 Free PMC article. Review. - Assessment of radiographic and prognostic characteristics of programmed death-ligand 1 expression in high-grade gliomas.
Ohno M, Kitano S, Satomi K, Yoshida A, Miyakita Y, Takahashi M, Yanagisawa S, Tamura Y, Ichimura K, Narita Y. Ohno M, et al. J Neurooncol. 2022 Nov;160(2):463-472. doi: 10.1007/s11060-022-04165-7. Epub 2022 Oct 25. J Neurooncol. 2022. PMID: 36282354 - Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue.
Bigelow E, Bever KM, Xu H, Yager A, Wu A, Taube J, Chen L, Jaffee EM, Anders RA, Zheng L. Bigelow E, et al. J Vis Exp. 2013 Jan 3;(71):4059. doi: 10.3791/4059. J Vis Exp. 2013. PMID: 23328703 Free PMC article.
References
- Benlalam H, Labarrière N, Linard B, Derré L, Diez E, Pandolfino MC, Bonneville M, Jotereau F. Comprehensive analysis of the frequency of recognition of melanoma-associated antigen (MAA) by CD8 melanoma infiltrating lymphocytes (TIL): Implications for immunotherapy. Eur. J. Immunol. 2001;31:2007–2015. - PubMed
- Kamposioras K, Pentheroudakis G, Pectasides D, Pavlidis N. Malignant melanoma of unknown primary site. To make the long story short. A systematic review of the literature. Crit. Rev. Oncol. Hematol. 2011;78:112–126. - PubMed
- Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008;8:467–477. - PubMed
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA97085/CA/NCI NIH HHS/United States
- R01 DK080736/DK/NIDDK NIH HHS/United States
- R01DK080736/DK/NIDDK NIH HHS/United States
- CA85721/CA/NCI NIH HHS/United States
- R01DK081417/DK/NIDDK NIH HHS/United States
- R01 DK081417/DK/NIDDK NIH HHS/United States
- CA016359/CA/NCI NIH HHS/United States
- R01 CA142779/CA/NCI NIH HHS/United States
- R01 CA163594/CA/NCI NIH HHS/United States
- R01 CA085721/CA/NCI NIH HHS/United States
- P30 CA006973/CA/NCI NIH HHS/United States
- R01 CA097085/CA/NCI NIH HHS/United States
- P30 CA016359/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials