Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains - PubMed (original) (raw)
Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains
Emmanuel Boucrot et al. Cell. 2012.
Abstract
Shallow hydrophobic insertions and crescent-shaped BAR scaffolds promote membrane curvature. Here, we investigate membrane fission by shallow hydrophobic insertions quantitatively and mechanistically. We provide evidence that membrane insertion of the ENTH domain of epsin leads to liposome vesiculation, and that epsin is required for clathrin-coated vesicle budding in cells. We also show that BAR-domain scaffolds from endophilin, amphiphysin, GRAF, and β2-centaurin limit membrane fission driven by hydrophobic insertions. A quantitative assay for vesiculation reveals an antagonistic relationship between amphipathic helices and scaffolds of N-BAR domains in fission. The extent of vesiculation by these proteins and vesicle size depend on the number and length of amphipathic helices per BAR domain, in accord with theoretical considerations. This fission mechanism gives a new framework for understanding membrane scission in the absence of mechanoenzymes such as dynamin and suggests how Arf and Sar proteins work in vesicle scission.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
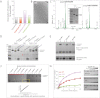
Predicted Membrane-Shaping Effect of Hydrophobic Insertions and Crescent-like Scaffolds (A) Computationally predicted membrane configurations generated out of an initially continuous flat membrane by a combined action of hydrophobic insertions and crescent-like scaffolds. The predictions are based on model computations (see Extended Experimental Procedures), and presented as phase diagrams for different ratios between the bending rigidities of a protein scaffold and a lipid monolayer, κp/κm, characterizing the relationship between membrane shaping powers of insertions and scaffolds. Small values of κp/κm correspond to a prevailing effect of insertions, whereas large κp/κm values correspond to a strong effect of scaffolds. The parameters describing the insertions and scaffolds and the number of insertions per scaffold are taken as for amphiphysin N-BAR domain (see Extended Experimental Procedures). The specific values of κp/κm describing the transitions between different configurations correspond to those predicted for the amphiphysin-like N-BAR domains at the protein-to-lipid molar ratio x = 0.003 (see extended phase diagram in Figure S1D). (B) Phase diagram for endophilin-like N-BAR module showing the ranges of the protein-to-lipid ratio, x, and the ratios κp/κm for which the initially flat membranes undergo bending and fission (vesicular state); bending without fission (tubular state), or coexistence of the two regimes (see Figure S1). (C) Predicted effects of the hydrophobic insertions (green wedges) and crescent-like scaffolds (red scaffolds) on a saddle-shaped membrane neck connecting two membranes. A saddle has both positive (red) and negative (orange) curvatures. The scaffolds stabilize the neck into a tubule and, hence, prevent fission. The insertions destabilize the saddle-like shape of the neck, hence favoring fission.
Figure 2
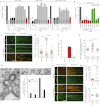
Epsin Is Required for CCV Scission (A) Effect of RNAi (siRNA pool1) of epsin proteins on transferrin (Tf) uptake measured by flow cytometry. Clathrin (CHC), AP2, and FCHo proteins depletion were used as positive controls (black bars). The values were normalized to the mean of the control cells (gray bars). The background (cells without Tf) for each cell line is shown (white bars). The number of cells analyzed is displayed on each bar. ∗∗∗p < 0.0001. Data are the mean ± SD. (B) Effects of 5 independent pools of siRNA against Epsin1+2+3 (red bars) on Tf uptake and of the rescue of pools 1 and 2 (but not CHC and AP2 RNAi) by coexpression of rat epsin1-RFP (green bars). Experiments were done as in (A). Data are the mean ± SD. (C) Effect of epsin1+2+3 RNAi on the dynamics of clathrin-coated structures (CCS) and rescue by coexpression of rat epsin1-RFP. CCS labeled by σ2-EGFP. Bar, 5 μm. (D) Scatter plots of individual lifetimes of CCS from three different cells, measured on data set similar to (C). Median with interquartile range is shown on graph and mean ± SD is written at the bottom, n is the number of events analyzed. ∗∗∗p < 0.0001. (E) Fraction of CCS with longer duration than the time series. (F) Scatter plots of individual maximum fluorescence intensities of CCS from three different cells. Data are presented as in (D), excepted for the Log10 vertical axis. ∗∗∗p < 0.0001. (G) Morphological analysis of CCS in BSC1 cells treated or not with epsin1+2+3 RNAi. Representative electron microscopy images for various categories quantified (top). Bars, 100 nm. Coated structures were classified as 1, shallow; 2, invaginated; 3, constricted; and 3∗, multiheaded. Repartition between the various categories of 70 structures from control (white bars) and 1+2+3 RNAi (black bars) cells is shown. Large image on left and 3∗ image are from RNAi-treated cells. (H) Effect of epsin1+2+3 RNAi on recruitment of endogenous dynamin 2 (DNM2en, green) and clathrin (CLTAen, red). Bar, 5 μm. (I) Scatter plots of individual lifetimes (top) and individual maximum fluorescence intensities (bottom) of endogenous dynamin2. Data are presented as in (D) and (F), respectively. See also Figure S2.
Figure 3
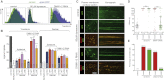
Epsin Can Mediate CCV Scission in Dynamin-Depleted Cells (A) Representative FACS profiles of transferrin (Tf) uptake in BSC1 cells treated with 80 μM dynasore or dynamin (DNM) 1+2 RNAi as in (B) and with (blue) or without (green) rat epsin1-RFP expression. (B) Effect of rat epsin1-RFP (wt), L6W and L6E mutants on Tf uptake or dextran uptake in cells treated with 80 μM dynasore or DNM1+2 RNAi. The values were normalized to the mean of the control cells (gray bars). The background (cells without Tf or dextran) is shown (white bars). Number of cells analyzed is displayed on each bar. ∗∗∗p < 0.0001, ∗∗p < 0.001. Data are the mean ± SD. (C) Effect of rat epsin1-RFP coexpression on CCS (labeled by σ2-EGFP) dynamics in cells treated with 80 μM dynasore or DNM1+2 RNAi as in (B). Bar, 5 μm. (D) Scatter plots of individual lifetimes of CCS from three different cells, measured on data sets similar to (C). Median with interquartile range is shown on graph and mean ± SD is written at the bottom, n is the number of events analyzed. ns, nonsignificant; ∗∗∗p < 0.0001. (E) Fraction of CCS with longer duration than the time series. See also Figure S3.
Figure 4
Epsin ENTH Domain Causes Extensive Membrane Vesiculation (A) Epsin (10 μM) incubated with 0.125 mg/ml Folch liposomes for 1 hr at room temperature. Samples for electron microscopy were taken before and after centrifugation. For sonicated liposomes a sample was subjected to probe sonication for 5 s. Samples for centrifugation were spun as indicated in the diagram. Pellets (P) were resuspended in the same volume of buffer as the supernatant (S). Lipids and proteins were visualized as described in Experimental Procedures. (B) Folch liposomes filtered to various sizes were subjected to centrifugation and the lipid distribution was assessed by SDS-PAGE. Samples for electron microscopy were taken before the spin. A more complete distribution of vesicle sizes is shown in Figure S4C. See also Figure S4.
Figure 5
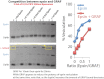
Membrane Vesiculation Is due to Amphipathic Helix Insertion (A) Membrane vesiculation due to epsin ENTH domain and mutants was assessed by the biochemical vesiculation assay and by electron microscopy. Protein (10 μM) was incubated with 0.125 mg/ml Folch + 5% PIP2 liposomes for 1 hr at 37°C. AP180 ANTH domain, which also binds to PIP2 (Ford et al., 2001), was used as a control. Data are mean of three experiments ± SD with a sample gel shown on the right. ∗∗p < 0.001. (B) Electron microscopy samples taken for samples in (A) after 5 min. (C) Quantitation of membrane vesiculation after 5 min incubation with WT and L6W epsin ENTH domain. The WT protein gives a broader distribution of vesicle sizes with many vesicles of larger diameters. Data in each case are from 169 objects in at least three different fields. One 200 nm vesicle is estimated to give 141 vesicles of 20 nm. (D) PIP2 dependence of epsin vesiculation. Epsin ENTH (10 μM) was incubated for 1 hr with either 200 nm or 30 nm-filtered synthetic liposomes (30% PS, 10% cholesterol, 55%–60% PC plus indicated amount of PIP2, final concentration of liposomes: 0.125 mg/ml). We see no effect of protein addition on the 30 nm-filtered liposomes. Vesiculation is dependent on PIP2, but binding can still be observed. (E) Limited trypsin proteolysis (20 min at 37°C) of epsin ENTH domain was inhibited by Soybean trypsin inhibitor (Inh.) or by liposomes (left). ∗For cleaved peptide sequence, see Figure S4C. The amphipathic helix was either pretrypsinized or not before addition of liposomes (right). (F) The amount of vesiculation shows a strong correlation with the amount of epsin protected, as assessed by a trypsin assay in (E). Thus it is not so important to know the amount of epsin added or membrane bound but the amount of helix insertion. See also Figure S5.
Figure 6
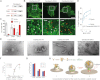
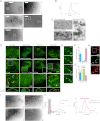
BAR Scaffolds Restrain Membrane Scission Catalyzed by Extensive Hydrophobic Insertions (A) Membrane tubulation and vesiculation by endophilins A1 and A3. Full-length proteins (4 μM) were incubated with liposomes at 0.5 mg/ml for 15 min at room temperature and then prepared for EM. (B) Vesiculation by Endo (full-length EndoA3), Amph (full-length amphiphysin 2–6), GRAF (GRAF1 BAR+PH domain), and Epsin (epsin1 ENTH domain). Folch liposomes at 0.125 mg/ml were incubated for 1 hr at 37°C. EndoA3 (2 μM), 4 μM Amph, and 8 μM GRAF was used (increasing concentrations were used to compensate for potentially reduced binding with less hydrophobic insertions). Data are the mean of three experiments ± SD. A sample gel is included. ∗∗p < 0.001. (C) Corresponding EMs of samples in (B) before sedimentation. Larger areas of the grids are shown in Figure S3. (D) Correlation between the extent of hydrophobic insertions and vesiculation. (E) Schematic representation of the endophilin mutants used. EndophilinA3 WT has an N-terminal amphipathic helix (red), a BAR domain (BAR), and a C-terminal SH3 domain (SH3). Endo-DAH has a Double N-terminal Amphipathic Helix. Endo-K4A4 and Endo-K8 have, respectively, four lysines (K4) and four alanines (4A) or eight lysines (K8), instead of their N-terminal amphipathic helices. Experiments were conducted with untagged proteins. (F) Membrane binding for WT and helix mutants. Protein (4 μM) was incubated for 15 min at room temperature with excess Folch liposomes to avoid vesiculation. Liposomes were added to the right two lanes in each panel. (G) Histogram showing the percentage of transfected cells displaying internal tubules (white) and internal vesicles (red). Cells could present both. Data are the mean ± SD of >300 cells for each construct from three independent experiments. ns, nonsignificant, ∗∗p < 0.001. (H) Vesiculation by Endo-WT DAH and K8. Liposomes (0.125 mg/ml) were incubated for 1 hr at 37°C with 2 μM protein. Data are the mean of three experiments ±SD. A sample gel is included. ∗∗p < 0.001. (I) EMs of samples taken before the sedimentation in (H). Larger areas of the grids are shown in Figure S6. (J) Graph showing the extent of vesiculation with different numbers of amphipathic helices. Data taken from (H) and (J). See also Figure S6.
Figure 7
Amphipathic Helix Addition to a BAR Scaffold Is Sufficient to Mediate Membrane Scission (A) Schematic representation of mutant centaurin proteins. β2-Centaurin WT BAR+PH domain had no amphipathic helices. Centaurin-SAH and centaurin-DAH had respectively a Single Amphipathic Helix or a Double Amphipathic Helix from EndoA3 at their N terminus. All constructs had a Myc-tag at the N terminus. (B) Confocal images of COS-7 cells expressing the BAR+PH domains of centaurin-WT, centaurin-SAH, or centaurin-DAH. The first row represents the maximal projection of a 3D stack of images acquired at 0.25 μm apart. The second row displays the insets of the boxed regions. Note the tubules (white arrows) and the internal vesicles (red arrows). Bar, 10 μm. (C) Histogram showing the percentage of transfected cells displaying internal tubules (white) and internal vesicles (red). Cells could present both. Data are the mean ± SD of >300 cells for each constructs from three independent experiments. ∗∗p < 0.001. (D) EM of liposomes with 9 μM of the indicated proteins. (E) Competition between epsin ENTH domain and β2-centaurin for vesiculation/tubulation of Folch liposomes. Mean ± SD for three independent experiments. Red bar: p < 0.001. (F) Predicted percentage of vesiculated membrane by N-BAR domains covering 50% of the total membrane area as a function of the total area of inclusions per scaffold Ains. Points represent the measured values in vitro and in vivo (Figure 6) for Endo-K8 (Ains = 7 nm2), Endo-WT (Ains = 20 nm2, and Endo-DAH (Ains = 32 nm2). (G) Predicted and measured diameters of vesicles generated as a result of membrane fission by Amph (Ains = 12 nm2, Endo-DAH (Ains = 32 nm2), and epsin ENTH domain (Ains = 6 nm2). In the computations 50% membrane coverage was used. (H) Model of the concentration of epsin to the region of membrane scission during CCV maturation. See also Figure S7.
Figure S1
Predicted Membrane-Shaping Effects of Hydrophobic Insertions and Crescent-like Scaffolds, Related to Figure 1 (A) Structures of Epsin ENTH domain (PDB: 1h0a), Amphiphysin BAR domain (PDB: 1uru), and endophilin BAR domain (PDB: 2c08) (Ford et al., 2002; Gallop et al., 2006; Peter et al., 2004). Epsin ribbon diagram is colored from N-C in red to magenta. Amphiphysin and endophilin monomer 1 is colored N-C in red to yellow and monomer 2 is colored in cyan to magenta. The amphipathic helix of epsin 1 ENTH domain is folded around the head group of PtdIns(4,5)P2 – Ins(3,4,5)P3. For amphiphysin and endophilin the terminal amphipathic helices are not present in the structures and so are connected to the structures by dotted lines. (B) Hydrophobic insertion mechanism. (C) Scaffolding mechanism. (D–F, upper panels) The free energies per lipid in the tubular (solid purple line) and vesicular (solid green line). The energies are plotted as a function of the protein-to-lipid ratio x for the effective protein rigidity κp = 4 · 10−19 Joule, and the monolayer modulus of Gaussian curvature κ¯=−2·10−21 Joule. The straight dashed lines indicate the common tangents to the energy curves determining the phase compositions at phase transitions. The in-plane area of hydrophobic insertions per one proteins scaffold Ains is taken to be (D) Ains = 12 nm2 for amphiphysin. (E) Ains = 20 nm2 or endophilin WT. (F) Ains = 30 nm2 for endophilin DAH. (D–F, lower panels). The phase diagrams showing the ranges of the protein-to-lipid ratio, x, and the ratios between the bending rigidities of a scaffold, κp, and a lipid monolayer,κm, for which the initially flat membranes undergo bending and fission (vesicular phase); bending without fission (tubular phase), or coexistence of the two regimes. The monolayer bending modulus and the modulus of Gaussian curvature are taken κ = 4 · 10−20 Joule and κ¯=−2·10−21 Joule, respectively, the effective spontaneous curvature of insertion is ζs = 0.75 nm-1. (G) Phase diagram taking onto account variations of the membrane modulus of Gaussian curvature κ¯B for endophilin WT with the total insertion area per scaffold Ains = 20 nm2. Other parameters are as in (D)–(F). (H) An illustration of the tilt angle of scaffolds on cylindrical vesicles. The tilt angle ψ is the angle that the elongated direction of the N-BAR scaffold (colorful coils) makes with the cylinder axis. The curvature of the scaffold at its center varies with the scaffold orientation, assuming values between R-1 and zero. (I) The orientation of the scaffolds in the tubular phase relative to the cylinder axis ψ as a function of the protein-to-lipid ratio x for amphiphysin. For low N-BAR concentrations the scaffolds are perpendicular to the axis ψ=π/2. Above the critical ratio x ≈0.0128, the orientation angle ψ begins to deviate from π/2.
Figure S2
Epsin Is Required for CCV Scission, Related to Figure 2 (A) Representative FACS profiles of transferrin uptake (20 μg/ml for 7 min at 37°C) in BSC1 cells treated with control, clathrin (CHC), AP2, FCHO1+2, or Epsin1+2+3 pool 1 siRNA (72 hr prior to ligand uptake) or Epsin1+2+3 pool 1 rescued by expression of rat epsin1-RFP (blue). (B) Effect of RNAi, using the siRNA pools 1 and 2, of epsin proteins on transferrin uptake (20 μg/ml for 7 min at 37°C) measured by flow cytometry. CHC, AP2, and FCHO protein depletions were used as positive controls (black bars). The values were normalized to the mean of the control cells (gray bars). The background (cells without transferrin) for each cell line is shown (white bars, background). Data are the mean ± SD. (C) Effect of RNAi, using the siRNA pool1 and 2, of epsin proteins on dextran uptake (1 mg/ml for 15 min at 37°C) measured by flow cytometry. AP2 depletion was used as positive controls (black bars). The values were normalized to the mean of the control cells (gray bars). Data are the mean ± SD. (D) Percentage of cells knocked down for the target proteins, as measured by the proportion of cells presenting a decrease in transferrin uptake down to background levels (‘P4’ region in A). Please note that less than half of the cells in FCHo1+2 and Epsin1+2+3 RNAi pools were strongly knocked down. Right, example of FACS profile of 2 populations (one strongly inhibited, ‘P4’ region, and another partially, ‘arrow’) in an Epsin1+2+3 RNAi sample. Data are the mean ± SD. (E) Effect of epsin1+2+3 RNAi on recruitment of rat dynamin1 (red) and AP2 (σ2-EGFP, green). Bar, 5 μm.
Figure S3
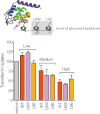
Epsin Can Mediate CCV Scission in Dynamin-Depleted Cells, Related to Figure 3 Top, scheme depicting the level of insertion of WT (leucine 6 in red) and L6W and L6E mutants. Bottom, effect of various Epsin1 WT, L6W, and L6E overexpression levels on transferrin uptake (20 μg/ml for 7 min at 37°C) measured by flow cytometry. ”Low,” “Medium,: and ”High” corresponded to 10 ng, 50 ng, and 200 ng of epsin-encoding DNA per 2 × 104 cells, respectively. Data are the mean ± SD.
Figure S4
Epsin ENTH Domain Causes Extensive Membrane Vesiculation, Related to Figure 4 (A) Surface charge of epsin ENTH domain. A highly positively charged region surrounded by hydrophobic surface residues acts as the binding site for the head group of PtdIns(4,5)P2 (PIP2). All ribbon diagrams and surface representations are to scale. (B) Model of how lipids must tilt and splay around the wedge-like shallow insertions of epsin amphipathic helix. Lipids are shown with red head groups and green acyl chains. A view directly down on the surface of the membrane gives a bird-eye view of the area covered by the ENTH domain. (C) Liposome size distribution after filtration to various diameters indicated. 100 liposomes for each category were measured and binned to the sizes indicated by the symbols. (D) Concentration dependence for epsin WT vesiculation of 0.125 mg/ml Folch liposomes at room temperature. Vesiculation is assessed as the percentage of lipid found in the supernatant after centrifugation. (E) Vesiculation by of a synthetic mixture of lipids containing 5% PtdIns(4,5)P2.
Figure S5
Membrane Vesiculation Is due to Amphipathic Helix Insertion, Related to Figure 5 (A) At 4°C epsin vesiculation is much less efficient. After 30 min incubation with 200 nm Folch liposomes only L6W is successful in giving a partial shift of liposomes from the pellet to the supernatant in the sedimentation assay. (B) Representative EM of liposomes after epsin L6W, used to quantitate the diameter of small vesicles shown in (A). Smaller particles (micelles or protein aggregates) were not counted. (C) Mass spectrometry of the complete trypsin digested sample of L6W epsin ENTH domain gave 2 prominent low molecular mass peptides (red box) whose sequence shows they are digested at 2 adjacent arginines. Arg8 coordinates the 4′ phosphate of the PIP2 inositol ring, whereas Arg7 coordinates the phosophodiester linkage (Ford et al., 2002). Removal of these residues by proteolysis means that the protein no longer binds membranes (Figure 2D). The mass of the proteolysed parent ENTH domain is consistent with a further cleavage of the amphipathic helix to the next lysine. This 3 amino acid peptide was not recovered. (D) Trypsin itself is not inhibited by membranes. The possibility that trypsin is absorbed/inhibited by membranes was tested by taking an unfolded protein (synaptobrevin) which gives distinct cleavage products (∗) and adding this to the liposome mixture. Proteins were preincubated with liposomes for 10 min before trypsin addition. Trypsin digestion of synaptobrevin is not inhibited by the addition of liposomes and may even be slightly enhanced. (E) Epsin binding to synthetic liposomes (30% PS, 10% cholesterol, 55%–60% PC) with various PIP2 contents. Samples were subjected to 15 min incubation with trypsin to eliminate uninserted protein, showing that the limited amount of protected protein in the presence of 0.5% PIP2 was sufficient to give significant vesiculation. (F) Increasing concentrations of epsin promote more extensive vesiculation of liposomes. After trypsin cleavage it becomes clear that, at higher concentrations, most of the added epsin is not bound/protected by the liposomes and so does not contribute to vesiculation. When vesiculation is plotted versus the bound/protected protein, vesiculation is linear relative to inserted epsin protein. The yellow box indicates the point of approximately 50% vesiculation where there is maximally 10% membrane coverage by epsin (assuming that 6ul epsin is giving saturation). (G) Membrane binding of epsin ENTH domain and mutants. It is not easy to assess the amount of epsin bound to membrane by the traditional sedimentation assay as the protein causes a shift in the liposomes sedimentation pattern. However given that amphipathic helix insertion is the major event one wants to monitor and is a reflection of binding, we can use trypsin sensitivity of the protein as a measure of insertion. At room temperature for 30 min L6W mutant binds better than WT protein, which binds better than L6E mutant protein.
Figure S6
BAR Scaffolds Restrain Membrane Scission Catalyzed by Extensive Hydrophobic Insertions, Related to Figure 6 (A) Electron micrographs of liposomes treated with various BAR-domain proteins. White boxes are the areas detailed in the corresponding main figure. (B) Size distribution of vesicles for amphiphysin2. Tubules and vesicles above 70 nm were excluded. (C) Examples of vesiculation by BAR domains taken from previous figures (Gallop et al., 2006; Peter et al., 2004). It had previously been noted that at higher concentrations D. melanogaster amphiphysin BAR domain tended to produce vesicles of a rather uniform size. (D) Confocal images of live HeLa cells expressing the respective endophilin constructs. First row represents the first focal plane at the bottom of the cells (“plasma membrane”). Note the presence of puncta with all four constructs. The second row represents a focal plane took 1 micron above the plasma membrane. The third row displays the inset of the boxed region. Note the tubules (white arrows) and the internal vesicles (red arrows). The last row shows a Z profile of each cell. (E) Time-lapse imaging of vesicle formation. EndophilinA3 tubule (white arrows) vesiculating and forming vesicle (red arrows). (F) Histogram reports the percentage of tubules that vesiculated for each constructs. (G) Histogram depicts the time (average ± standard error of the mean [SEM]) to vesiculation of 50 tubules for each constructs (10 tubules for K4A4 constructs as they were rare). The tubules formed by the K4A4 and K8 constructs were stable and did not vesiculate during the time of imaging (600 s). Significance determined using Student's t test (∗∗p < 0.001). (H) Electron micrographs of liposomes treated with various endophilin constructs (Endo-WT and mutants of the N-terminal amphipathic helix, DAH, double amphipathic helix and K8, N-terminal helix replaced by a stretch of 8 lysines). Liposomes were incubated for 60 min at 37°C with 2 μM protein. White boxes are the areas detailed in the corresponding main figure. (I) Size distribution of vesicles for Endo-DAH (pink) and starting liposomes (gray).
Figure S7
GRAF BAR+PH Domain Resists Epsin Vesiculation Activity, Related to Figure 7 Competition between epsin ENTH domain, which promotes vesiculation, and GRAF BAR+PH domain, which promotes membrane tubulation. Although GRAF does indeed appear to restrain the vesiculation, this effect can be accounted for by the reduced binding of epsin in the presence of GRAF.
Comment in
- Membrane fission: curvature-sensitive proteins cut it both ways.
Callan-Jones A, Bassereau P. Callan-Jones A, et al. Dev Cell. 2012 Apr 17;22(4):691-2. doi: 10.1016/j.devcel.2012.04.001. Dev Cell. 2012. PMID: 22516194
Similar articles
- Single particle fluorescence burst analysis of epsin induced membrane fission.
Brooks A, Shoup D, Kustigian L, Puchalla J, Carr CM, Rye HS. Brooks A, et al. PLoS One. 2015 Mar 23;10(3):e0119563. doi: 10.1371/journal.pone.0119563. eCollection 2015. PLoS One. 2015. PMID: 25799353 Free PMC article. - Molecular basis of the potent membrane-remodeling activity of the epsin 1 N-terminal homology domain.
Yoon Y, Tong J, Lee PJ, Albanese A, Bhardwaj N, Källberg M, Digman MA, Lu H, Gratton E, Shin YK, Cho W. Yoon Y, et al. J Biol Chem. 2010 Jan 1;285(1):531-40. doi: 10.1074/jbc.M109.068015. Epub 2009 Nov 1. J Biol Chem. 2010. PMID: 19880963 Free PMC article. - Understanding the role of amphipathic helices in N-BAR domain driven membrane remodeling.
Cui H, Mim C, Vázquez FX, Lyman E, Unger VM, Voth GA. Cui H, et al. Biophys J. 2013 Jan 22;104(2):404-11. doi: 10.1016/j.bpj.2012.12.006. Biophys J. 2013. PMID: 23442862 Free PMC article. - BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature.
Itoh T, De Camilli P. Itoh T, et al. Biochim Biophys Acta. 2006 Aug;1761(8):897-912. doi: 10.1016/j.bbalip.2006.06.015. Epub 2006 Jul 28. Biochim Biophys Acta. 2006. PMID: 16938488 Review. - A unifying mechanism accounts for sensing of membrane curvature by BAR domains, amphipathic helices and membrane-anchored proteins.
Bhatia VK, Hatzakis NS, Stamou D. Bhatia VK, et al. Semin Cell Dev Biol. 2010 Jun;21(4):381-90. doi: 10.1016/j.semcdb.2009.12.004. Epub 2009 Dec 16. Semin Cell Dev Biol. 2010. PMID: 20006726 Review.
Cited by
- GTP-stimulated membrane fission by the N-BAR protein AMPH-1.
Kustigian L, Gong X, Gai W, Thongchol J, Zhang J, Puchalla J, Carr CM, Rye HS. Kustigian L, et al. Traffic. 2023 Jan;24(1):34-47. doi: 10.1111/tra.12875. Epub 2022 Dec 13. Traffic. 2023. PMID: 36435193 Free PMC article. - Spatial Control of Epsin-induced Clathrin Assembly by Membrane Curvature.
Holkar SS, Kamerkar SC, Pucadyil TJ. Holkar SS, et al. J Biol Chem. 2015 Jun 5;290(23):14267-76. doi: 10.1074/jbc.M115.653394. Epub 2015 Apr 2. J Biol Chem. 2015. PMID: 25837255 Free PMC article. - Membrane fission by dynamin: what we know and what we need to know.
Antonny B, Burd C, De Camilli P, Chen E, Daumke O, Faelber K, Ford M, Frolov VA, Frost A, Hinshaw JE, Kirchhausen T, Kozlov MM, Lenz M, Low HH, McMahon H, Merrifield C, Pollard TD, Robinson PJ, Roux A, Schmid S. Antonny B, et al. EMBO J. 2016 Nov 2;35(21):2270-2284. doi: 10.15252/embj.201694613. Epub 2016 Sep 26. EMBO J. 2016. PMID: 27670760 Free PMC article. Review. - Dynamin: membrane scission meets physics.
Hurley JH, Hinshaw JE. Hurley JH, et al. Curr Biol. 2012 Dec 18;22(24):R1047-8. doi: 10.1016/j.cub.2012.11.008. Curr Biol. 2012. PMID: 23257190 Free PMC article. - Membrane effects of N-terminal fragment of apolipoprotein A-I: a fluorescent probe study.
Trusova V, Gorbenko G, Girych M, Adachi E, Mizuguchi C, Sood R, Kinnunen P, Saito H. Trusova V, et al. J Fluoresc. 2015 Mar;25(2):253-61. doi: 10.1007/s10895-015-1501-9. Epub 2015 Jan 18. J Fluoresc. 2015. PMID: 25595057
References
- Antonny B. Membrane deformation by protein coats. Curr. Opin. Cell Biol. 2006;18:386–394. - PubMed
Supplemental References
- Ehrlich, M., Boll, W., Van Oijen, A., Hariharan, R., Chandran, K., Nibert, M.L., and Kirchhausen, T. (2004). Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 118, 591–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous