Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer - PubMed (original) (raw)
doi: 10.1136/gutjnl-2012-302529. Epub 2012 Mar 30.
Derek S Chan, Albrecht Neesse, Tashinga E Bapiro, Natalie Cook, Kristopher K Frese, Christine Feig, Tomoaki Nakagawa, Meredith E Caldwell, Heather I Zecchini, Martijn P Lolkema, Ping Jiang, Anne Kultti, Curtis B Thompson, Daniel C Maneval, Duncan I Jodrell, Gregory I Frost, H M Shepard, Jeremy N Skepper, David A Tuveson
Affiliations
- PMID: 22466618
- PMCID: PMC3551211
- DOI: 10.1136/gutjnl-2012-302529
Free PMC article
Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer
Michael A Jacobetz et al. Gut. 2013 Jan.
Free PMC article
Abstract
Objective: Pancreatic ductal adenocarcinoma (PDA) is characterised by stromal desmoplasia and vascular dysfunction, which critically impair drug delivery. This study examines the role of an abundant extracellular matrix component, the megadalton glycosaminoglycan hyaluronan (HA), as a novel therapeutic target in PDA.
Methods: Using a genetically engineered mouse model of PDA, the authors enzymatically depleted HA by a clinically formulated PEGylated human recombinant PH20 hyaluronidase (PEGPH20) and examined tumour perfusion, vascular permeability and drug delivery. The preclinical utility of PEGPH20 in combination with gemcitabine was assessed by short-term and survival studies.
Results: PEGPH20 rapidly and sustainably depleted HA, inducing the re-expansion of PDA blood vessels and increasing the intratumoral delivery of two chemotherapeutic agents, doxorubicin and gemcitabine. Moreover, PEGPH20 triggered fenestrations and interendothelial junctional gaps in PDA tumour endothelia and promoted a tumour-specific increase in macromolecular permeability. Finally, combination therapy with PEGPH20 and gemcitabine led to inhibition of PDA tumour growth and prolonged survival over gemcitabine monotherapy, suggesting immediate clinical utility.
Conclusions: The authors demonstrate that HA impedes the intratumoral vasculature in PDA and propose that its enzymatic depletion be explored as a means to improve drug delivery and response in patients with pancreatic cancer.
Conflict of interest statement
Competing interests: DCM, CBT, PJ, AK, HMS and GIF are employees of Halozyme.
Figures
Figure 1
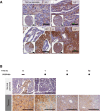
Hyaluronan (HA) accumulates in pancreatic ductal adenocarcinoma (PDA) and may be rapidly and sustainably degraded by PEGPH20. (A) HA expression in human pancreatic cancer samples (i–iii) varies from low expression (HA1+) to high expression (HA3+), with little HA detected in normal pancreas (top left panel) (scale bar: inset 300 μm, 100 μm). (B) HA expression in normal pancreas and PDA over time after treatment with PEGPH20. PEGPH20 treatment specifically degrades HA in normal pancreas (top row) 1 h after treatment. Maximal HA degradation was achieved in KPC tumours 8 h after PEGPH20 treatment that still remained low even 72 h after treatment. n=3 mice per time point (scale bar: 200 μm).
Figure 2
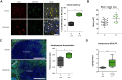
Depletion of hyaluronan improves vascular patency and increases chemotherapeutic delivery. (A) Fluorescence images of KPC tumours from vehicle-treated (top row, V) and PEGPH20-treated (bottom row, P) mice (n=4 animals in each cohort) terminally perfused with fluorescent biotinylated lectin (red). Total blood vessels are detected with MECA-32 antibody (green). White arrows denote functional vessels (red and green). Yellow arrows denote non-functional vessels (green only). Quantification of vessel patency indicates a significant increase in functional vessels after treatment with PEGPH20 (n=4 mice in each cohort, >100 vessels per mouse). Scale bar = 100 μm. Mann–Whitney U test, *p=0.0286. (B) Quantification of mean vessel area (in square micrometres) from anti-CD31-stained KPC tumours from vehicle-treated (V) or PEGPH20-treated (P) mice. A significant increase in mean vessel area is observed after treatment with PEGPH20, Mann–Whitney U test, **p=0.0085. (C) Fluorescent image of representative KPC tumour from vehicle-treated (top panel) and PEGPH20-treated (bottom panel) mice terminally perfused with autofluorescent small-molecule doxorubicin (pseudo-coloured green, n=4 mice in each cohort). Scale bar = 1 mm. Dotted white line denotes border between tumour core and adjacent diseased pancreatic tissue. Quantification of intratumoral doxorubicin as measured by fluorescence ratio in vehicle-treated (V) and PEGPH20-treated (P) KPC mice pancreatic ductal adenocarcinoma. Significant increase in intratumoral doxorubicin in PEGPH20-treated samples, Mann–Whitney U test, *p=0.0260. (D) Quantification of intratumoral gemcitabine triphosphate (dFdCTP), the active metabolite of gemcitabine (G), from vehicle- and PEGPH20-pretreated KPC mice. Significant increase in dFdCTP in PEGPH20-treated tumours, Mann–Whitney U test, **p=0.0053.
Figure 3
Depletion of hyaluronan increases macromolecular permeability and induces ultrastructural changes in tumour endothelia. (A) Representative fluorescent images of KPC tumour from vehicle-treated (top panel) and PEGPH20-treated (bottom panel) mice (n=4 mice for each cohort). Mice terminally treated with either low (40 kDa) or high (2 MDa) molecular weight dextrans with biotinylated lectin demonstrate a considerable increase in stromal and vessel permeability at the tumour core in PEGPH20-treated tumours. Arrows denote functional vessels in the field. Scale bar = 500 μm. (B) Scanning electron microscopy images of pancreatic blood vessels in PC (LSL-Trp53R172H/+;Pdx-1-Cre) (upper two panels) and KPC (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre) (lower two panels) mice following treatment with either vehicle or PEGPH20 (n=4 mice for each cohort) reveal endothelial fenestrations (white arrowheads) only in PEGPH20-treated KPC mice. (C) Quantification of density of fenestrae in intratumoral KPC blood vessels (n=3 mice for each cohort, four random vessels per mouse) revealed a significant increase following treatment with PEGPH20 (***p<0.0001). (D) Representative transmission electron micrographs from vehicle-treated (left panels) and PEGPH20-treated (right panels) tumours (n=4 mice for each cohort). Vehicle-treated tumours demonstrate juxtaposed endothelial cell membranes (red arrowheads), while prominent interendothelial gaps are present following treatment with PEGPH20 (designated by white diamonds, endothelial cell membranes denoted by blue arrowheads).
Figure 3
Depletion of hyaluronan increases macromolecular permeability and induces ultrastructural changes in tumour endothelia. (A) Representative fluorescent images of KPC tumour from vehicle-treated (top panel) and PEGPH20-treated (bottom panel) mice (n=4 mice for each cohort). Mice terminally treated with either low (40 kDa) or high (2 MDa) molecular weight dextrans with biotinylated lectin demonstrate a considerable increase in stromal and vessel permeability at the tumour core in PEGPH20-treated tumours. Arrows denote functional vessels in the field. Scale bar = 500 μm. (B) Scanning electron microscopy images of pancreatic blood vessels in PC (LSL-Trp53R172H/+;Pdx-1-Cre) (upper two panels) and KPC (LSL-KrasG12D/+;LSL-Trp53R172H/+;Pdx-1-Cre) (lower two panels) mice following treatment with either vehicle or PEGPH20 (n=4 mice for each cohort) reveal endothelial fenestrations (white arrowheads) only in PEGPH20-treated KPC mice. (C) Quantification of density of fenestrae in intratumoral KPC blood vessels (n=3 mice for each cohort, four random vessels per mouse) revealed a significant increase following treatment with PEGPH20 (***p<0.0001). (D) Representative transmission electron micrographs from vehicle-treated (left panels) and PEGPH20-treated (right panels) tumours (n=4 mice for each cohort). Vehicle-treated tumours demonstrate juxtaposed endothelial cell membranes (red arrowheads), while prominent interendothelial gaps are present following treatment with PEGPH20 (designated by white diamonds, endothelial cell membranes denoted by blue arrowheads).
Figure 4
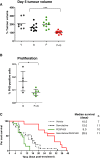
Combination therapy with PEGPH20 and gemcitabine inhibits tumour growth and significantly extends survival. (A) Tumour volume measurements from mice treated for 5 days with vehicle (V) (n=7), gemcitabine (G) (n=11), PEGPH20 (P) (n=10) and gemcitabine/PEGPH20 combination (P+G) (n=11) demonstrate cytostasis only in response to gemcitabine/PEGPH20 combination therapy. (B) Proliferation of pancreatic tumour cells in response to PEGPH20 treatment. A significant decrease in pancreatic tumour cell proliferation is observed 5 days after treatment with PEGPH20 and gemcitabine compared with gemcitabine alone. Mann–Whitney U test, *p=0.0432. (C) Kaplan–Meier survival curve for vehicle-treated (n=8, grey dotted line), gemcitabine-treated (n=15, black dotted line), PEGPH20-treated (n=12, green line) and gemcitabine/PEGPH20-treated (n=10, red line) KPC cohorts. A significant increase in survival was observed in gemcitabine/PEGPH20-treated mice relative to the gemcitabine-treated cohort. Log-rank ***p=0.0002, HR 0.06794. PH3, phospho-histone H3.
Similar articles
- Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma.
Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Provenzano PP, et al. Cancer Cell. 2012 Mar 20;21(3):418-29. doi: 10.1016/j.ccr.2012.01.007. Cancer Cell. 2012. PMID: 22439937 Free PMC article. - HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma.
Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. Hingorani SR, et al. J Clin Oncol. 2018 Feb 1;36(4):359-366. doi: 10.1200/JCO.2017.74.9564. Epub 2017 Dec 12. J Clin Oncol. 2018. PMID: 29232172 Clinical Trial. - Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid.
Michl P, Gress TM. Michl P, et al. Gut. 2012 Oct;61(10):1377-9. doi: 10.1136/gutjnl-2012-302604. Epub 2012 Jun 3. Gut. 2012. PMID: 22661496 No abstract available. - Targeting the Tumor Stroma: the Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20).
Wong KM, Horton KJ, Coveler AL, Hingorani SR, Harris WP. Wong KM, et al. Curr Oncol Rep. 2017 Jul;19(7):47. doi: 10.1007/s11912-017-0608-3. Curr Oncol Rep. 2017. PMID: 28589527 Review. - Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies.
Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Heinemann V, et al. Cancer Treat Rev. 2014 Feb;40(1):118-28. doi: 10.1016/j.ctrv.2013.04.004. Epub 2013 Jul 9. Cancer Treat Rev. 2014. PMID: 23849556 Review.
Cited by
- Depletion of Macrophages Improves Therapeutic Response to Gemcitabine in Murine Pancreas Cancer.
Buchholz SM, Goetze RG, Singh SK, Ammer-Herrmenau C, Richards FM, Jodrell DI, Buchholz M, Michl P, Ellenrieder V, Hessmann E, Neesse A. Buchholz SM, et al. Cancers (Basel). 2020 Jul 20;12(7):1978. doi: 10.3390/cancers12071978. Cancers (Basel). 2020. PMID: 32698524 Free PMC article. - The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment.
Ni Y, Zhou X, Yang J, Shi H, Li H, Zhao X, Ma X. Ni Y, et al. Front Cell Dev Biol. 2021 May 20;9:637675. doi: 10.3389/fcell.2021.637675. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34095111 Free PMC article. Review. - Intravital imaging reveals new ancillary mechanisms co-opted by cancer cells to drive tumor progression.
Vennin C, Herrmann D, Lucas MC, Timpson P. Vennin C, et al. F1000Res. 2016 May 16;5:F1000 Faculty Rev-892. doi: 10.12688/f1000research.8090.1. eCollection 2016. F1000Res. 2016. PMID: 27239290 Free PMC article. Review. - Use of a lipid-coated mesoporous silica nanoparticle platform for synergistic gemcitabine and paclitaxel delivery to human pancreatic cancer in mice.
Meng H, Wang M, Liu H, Liu X, Situ A, Wu B, Ji Z, Chang CH, Nel AE. Meng H, et al. ACS Nano. 2015;9(4):3540-57. doi: 10.1021/acsnano.5b00510. Epub 2015 Mar 31. ACS Nano. 2015. PMID: 25776964 Free PMC article. - Role of cancer-associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer.
Mhaidly R, Mechta-Grigoriou F. Mhaidly R, et al. Immunol Rev. 2021 Jul;302(1):259-272. doi: 10.1111/imr.12978. Epub 2021 May 19. Immunol Rev. 2021. PMID: 34013544 Free PMC article. Review.
References
- Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403–13 - PubMed
- Sultana A, Smith CT, Cunningham D, et al. Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 2007;25:2607–15 - PubMed
- Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 2005;7:469–83 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical