The immunology of neurodegeneration - PubMed (original) (raw)
Review
. 2012 Apr;122(4):1156-63.
doi: 10.1172/JCI58656. Epub 2012 Apr 2.
Affiliations
- PMID: 22466657
- PMCID: PMC3315444
- DOI: 10.1172/JCI58656
Review
The immunology of neurodegeneration
Eva Czirr et al. J Clin Invest. 2012 Apr.
Abstract
While immune responses in neurodegeneration were regarded as little more than a curiosity a decade ago, they are now increasingly moving toward center stage. Factors driving this movement include the recognition that most of the relevant immune molecules are produced within the brain, that microglia are proficient immune cells shaping neuronal circuitry and fate, and that systemic immune responses affect brain function. We will review this complex field from the perspective of neurons, extra-neuronal brain cells, and the systemic environment and highlight the possibility that cell intrinsic innate immune molecules in neurons may function in neurodegenerative processes.
Figures
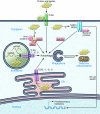
Figure 2. Innate immune receptors as sensors of intraneuronal distress.
Neurons express innate immune receptors that serve as sensors of danger signals. TLRs may recognize endogenous molecules and protein aggregates such as Aβ assemblies, ssRNA, or dsRNA aside from molecules associated with pathogens. While TLR4 and its co-receptor CD14 are present at the cell surface, TLR3, -7, -8, and -9 are located in the ER and endosomal compartments. Activation of TLRs can lead to initiation of autophagy via TRIF/RIP1 and possibly induce the clearance of defective organelles or protein aggregates. Activation of NOD1 or NOD2 may result in NF-κB–mediated transcription of pro-inflammatory genes or initiation of autophagy via Atg16L. The adaptor protein p62 can detect viral proteins in neurons and initiate clearance of viral particles via autophagy involving Atg5 and Atg7. It may also assist in the clearance of abnormal protein aggregates. Atg, autophagy-related protein; NOD, nucleotide binding oligomerization domain-like; p62, nucleoporin 62; TRIF, TIR domain–containing adapter-inducing IFN-β; RIP1, receptor interacting protein-1.
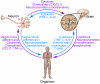
Figure 1. Immune-mediated interactions in neurodegeneration at the cellular, tissue, and systemic level.
Immune interactions in neurodegeneration can occur within neurons, in the brain tissue, or in the systemic environment through cytokines, neurotransmitters, cell-cell interactions, and peripheral nerves. Abnormalities at any of these levels of organization may modulate neurodegenerative processes in the CNS and may also serve as targets for therapeutic interventions. Some of the factors discussed in more detail in the text are listed in the figure.
Similar articles
- Toll-like receptors in the pathogenesis of neuroinflammation.
Kumar V. Kumar V. J Neuroimmunol. 2019 Jul 15;332:16-30. doi: 10.1016/j.jneuroim.2019.03.012. Epub 2019 Mar 20. J Neuroimmunol. 2019. PMID: 30928868 Review. - Harnessing immune alterations in neurodegenerative diseases.
Björkqvist M, Wild EJ, Tabrizi SJ. Björkqvist M, et al. Neuron. 2009 Oct 15;64(1):21-4. doi: 10.1016/j.neuron.2009.09.034. Neuron. 2009. PMID: 19840543 - Neuroimmune Crosstalk through Extracellular Vesicles in Health and Disease.
Delpech JC, Herron S, Botros MB, Ikezu T. Delpech JC, et al. Trends Neurosci. 2019 May;42(5):361-372. doi: 10.1016/j.tins.2019.02.007. Epub 2019 Mar 26. Trends Neurosci. 2019. PMID: 30926143 Free PMC article. Review. - Brain-immune connection: immuno-regulatory properties of CNS-resident cells.
Becher B, Prat A, Antel JP. Becher B, et al. Glia. 2000 Feb 15;29(4):293-304. Glia. 2000. PMID: 10652440 Review. - Microglia-Specific Metabolic Changes in Neurodegeneration.
Aldana BI. Aldana BI. J Mol Biol. 2019 Apr 19;431(9):1830-1842. doi: 10.1016/j.jmb.2019.03.006. Epub 2019 Mar 13. J Mol Biol. 2019. PMID: 30878483 Review.
Cited by
- Aspirin-triggered lipoxin A4 stimulates alternative activation of microglia and reduces Alzheimer disease-like pathology in mice.
Medeiros R, Kitazawa M, Passos GF, Baglietto-Vargas D, Cheng D, Cribbs DH, LaFerla FM. Medeiros R, et al. Am J Pathol. 2013 May;182(5):1780-9. doi: 10.1016/j.ajpath.2013.01.051. Epub 2013 Mar 16. Am J Pathol. 2013. PMID: 23506847 Free PMC article. - TLR7 negatively regulates dendrite outgrowth through the Myd88-c-Fos-IL-6 pathway.
Liu HY, Hong YF, Huang CM, Chen CY, Huang TN, Hsueh YP. Liu HY, et al. J Neurosci. 2013 Jul 10;33(28):11479-93. doi: 10.1523/JNEUROSCI.5566-12.2013. J Neurosci. 2013. PMID: 23843519 Free PMC article. - Allergy Enhances Neurogenesis and Modulates Microglial Activation in the Hippocampus.
Klein B, Mrowetz H, Thalhamer J, Scheiblhofer S, Weiss R, Aigner L. Klein B, et al. Front Cell Neurosci. 2016 Jun 28;10:169. doi: 10.3389/fncel.2016.00169. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27445696 Free PMC article. - Alzheimer's disease risk alleles in TREM2 illuminate innate immunity in Alzheimer's disease.
Golde TE, Streit WJ, Chakrabarty P. Golde TE, et al. Alzheimers Res Ther. 2013 May 21;5(3):24. doi: 10.1186/alzrt178. eCollection 2013. Alzheimers Res Ther. 2013. PMID: 23692967 Free PMC article. Review. - Post-treatment with an ultra-low dose of NADPH oxidase inhibitor diphenyleneiodonium attenuates disease progression in multiple Parkinson's disease models.
Wang Q, Qian L, Chen SH, Chu CH, Wilson B, Oyarzabal E, Ali S, Robinson B, Rao D, Hong JS. Wang Q, et al. Brain. 2015 May;138(Pt 5):1247-62. doi: 10.1093/brain/awv034. Epub 2015 Feb 25. Brain. 2015. PMID: 25716193 Free PMC article.
References
- Britschgi M, Wyss-Coray T. Systemic and acquired immune responses in Alzheimer’s disease. Int Rev Neurobiol. 2007;82:205–233. - PubMed
- Akiyama H, Arai T, Kondo H, Tanno E, Haga C, Ikeda K. Cell mediators of inflammation in the Alzheimer disease brain. Alzheimer Dis Assoc Disord. 2000;14 suppl 1:S47–S53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical