Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro - PubMed (original) (raw)
doi: 10.1186/1756-9966-31-32.
Cátia Monteiro, Virgínia Cunha, Maria José Oliveira, Mariana Freitas, Avelino Fraga, Paulo Príncipe, Carlos Lobato, Francisco Lobo, António Morais, Vítor Silva, José Sanches-Magalhães, Jorge Oliveira, Francisco Pina, Anabela Mota-Pinto, Carlos Lopes, Rui Medeiros
Affiliations
- PMID: 22469146
- PMCID: PMC3379940
- DOI: 10.1186/1756-9966-31-32
Human periprostatic adipose tissue promotes prostate cancer aggressiveness in vitro
Ricardo Ribeiro et al. J Exp Clin Cancer Res. 2012.
Abstract
Background: Obesity is associated with prostate cancer aggressiveness and mortality. The contribution of periprostatic adipose tissue, which is often infiltrated by malignant cells, to cancer progression is largely unknown. Thus, this study aimed to determine if periprostatic adipose tissue is linked with aggressive tumor biology in prostate cancer.
Methods: Supernatants of whole adipose tissue (explants) or stromal vascular fraction (SVF) from paired fat samples of periprostatic (PP) and pre-peritoneal visceral (VIS) anatomic origin from different donors were prepared and analyzed for matrix metalloproteinases (MMPs) 2 and 9 activity. The effects of those conditioned media (CM) on growth and migration of hormone-refractory (PC-3) and hormone-sensitive (LNCaP) prostate cancer cells were measured.
Results: We show here that PP adipose tissue of overweight men has higher MMP9 activity in comparison with normal subjects. The observed increased activities of both MMP2 and MMP9 in PP whole adipose tissue explants, likely reveal the contribution of adipocytes plus stromal-vascular fraction (SVF) as opposed to SVF alone. MMP2 activity was higher for PP when compared to VIS adipose tissue. When PC-3 cells were stimulated with CM from PP adipose tissue explants, increased proliferative and migratory capacities were observed, but not in the presence of SVF. Conversely, when LNCaP cells were stimulated with PP explants CM, we found enhanced motility despite the inhibition of proliferation, whereas CM derived from SVF increased both cell proliferation and motility. Explants culture and using adipose tissue of PP origin are most effective in promoting proliferation and migration of PC-3 cells, as respectively compared with SVF culture and using adipose tissue of VIS origin. In LNCaP cells, while explants CM cause increased migration compared to SVF, the use of PP adipose tissue to generate CM result in the increase of both cellular proliferation and migration.
Conclusions: Our findings suggest that the PP depot has the potential to modulate extra-prostatic tumor cells' microenvironment through increased MMPs activity and to promote prostate cancer cell survival and migration. Adipocyte-derived factors likely have a relevant proliferative and motile role.
Figures
Figure 1
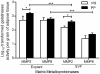
Gelatinolytic activity of periprostatic (PP) adipose tissue and comparison with visceral pre-peritoneal fat depot. Analyses were performed in explants and stromal-vascular fraction primary culture of 21 samples of PP adipose tissue and 10 samples of VIS adipose tissue. Independent samples _t_-test was used. *** P < 0.0001 between explants and SVF fraction; * P < 0.05 in the comparison among fat depots. MMP, matrix metalloproteinase; VIS, visceral; PP, periprostatic; SVF, stromal-vascular fraction.
Figure 2
MMP2 and MMP9 enzymatic activities in supernatants of whole adipose tissue and SVF fraction from VIS and PP depots. Representative bands corresponding to specific MMP2 and MMP9 are shown. Asterisks indicate active forms of MMP2 and MMP9 while arrows indicate the respective proforms. SVF, stromal-vascular fraction; PP, periprostatic; VIS, visceral; MMP, matrix metalloproteinase.
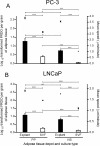
Figure 3
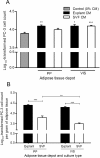
Influence of conditioned medium from distinct adipose tissue origins in the proliferation of PC-3 cells. Analyses were performed using conditioned medium of 21 samples of periprostatic (PP) and 10 samples of visceral (VIS) adipose tissue, after explants and stromal-vascular fraction primary cultures. A. Effect of adipose tissue-derived CM on PC-3 cell proliferation, in comparison with control (0% CM) (**P < 0.01 in relation with 0% CM, one-way ANOVA with two-sided post-hoc Dunnett test). B. PC-3 cell proliferation was normalized per gram of adipose tissue and compared according to fat depot and adipose tissue fraction (**P < 0.01 and *** P < 0.0001 between groups, independent samples _t_-test). CM, conditioned medium; PP, periprostatic; SVF, stromal-vascular fraction; VIS, visceral.
Figure 4
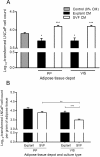
Influence of conditioned medium from adipose tissue in the proliferation of LNCaP cells. Analyses were conducted using conditioned medium of periprostatic (PP) and visceral (VIS) adipose tissue from 10 subjects after explants and stromal-vascular fraction primary cultures. A. Influence of adipose tissue-derived CM in LNCaP cell proliferation, in comparison with control (0% CM) (* P < 0.05 and ** P < 0.01, relative to control, two-sided post-hoc Dunnett test). B. Comparison of the effect of CM from distinct adipose tissue depot and fractions in LNCaP proliferation after tissue weight normalization (** P < 0.01 and *** P < 0.0001 between groups, independent samples _t_-test). CM, conditioned medium. SVF, stromal-vascular fraction. PP, periprostatic; VIS, visceral.
Figure 5
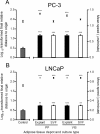
Motility of PC3 and LNCaP cells upon stimulation of adipose tissue-derived CM from explants and SVF. Influence of adipose tissue fractions in cell motility parameters. Data represent mean ± SE of at least 20 representative cell trajectories per each tested condition, with conditioned medium of primary adipose tissue cultures from four distinct subjects. Bars represent mean speed (MS) and plots the logarithmically transformed final relative distance to origin (FRDO). A. FRDO and MS of PC-3 cells (*** P < 0.0001 relative to control). B. FRDO and MS of LNCaP cells (** P < 0.01 and *** P < 0.0001 relative to control). In the log-transformed FRDO we used one-way ANOVA with post-hoc Dunnett test (two-sided), whereas the mean speed was analyzed using Kruskal Wallis followed by Mann Whitney test. SVF, stromal-vascular fraction; PP, periprostatic; VIS, visceral.
Figure 6
Motility of PC-3 and LNCaP cells upon stimulation of adipose tissue-derived CM from explants and SVF. Data represent mean ± SE of at least 20 representative cell trajectories per each tested condition, from four distinct subjects. Bars represent mean speed (MS) per gram of adipose tissue and plots the logarithmically transformed final relative distance to origin per gram of adipose tissue (FRDO). A. FRDO and MS of PC-3 cells (* P < 0.05 and *** P < 0.0001 between treatment conditions). B. FRDO and MS of LNCaP cells (** P < 0.01 and *** P < 0.0001 between conditions). Analyses on MS were performed with Mann Whitney test, whereas FRDO was analyzed using independent samples _t_-test.SVF, stromal-vascular fraction; PP, periprostatic; VIS, visceral.
Figure 7
Representative example of cell tracking and cancer cell trajectories after stimulation with periprostatic adipose tissue-derived CM. Sequential displacements of cells were captured by manual cell tracking and are represented as color lines. SVF, stromal-vascular fraction.
Similar articles
- Tumor cell-educated periprostatic adipose tissue acquires an aggressive cancer-promoting secretory profile.
Ribeiro RJ, Monteiro CP, Cunha VF, Azevedo AS, Oliveira MJ, Monteiro R, Fraga AM, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Guimarães JT, Lopes CM, Medeiros RM. Ribeiro RJ, et al. Cell Physiol Biochem. 2012;29(1-2):233-40. doi: 10.1159/000337604. Epub 2012 Mar 1. Cell Physiol Biochem. 2012. PMID: 22415092 - Obesity and prostate cancer: gene expression signature of human periprostatic adipose tissue.
Ribeiro R, Monteiro C, Catalán V, Hu P, Cunha V, Rodríguez A, Gómez-Ambrosi J, Fraga A, Príncipe P, Lobato C, Lobo F, Morais A, Silva V, Sanches-Magalhães J, Oliveira J, Pina F, Lopes C, Medeiros R, Frühbeck G. Ribeiro R, et al. BMC Med. 2012 Sep 25;10:108. doi: 10.1186/1741-7015-10-108. BMC Med. 2012. PMID: 23009291 Free PMC article. - MMP9 expression in oesophageal adenocarcinoma is upregulated with visceral obesity and is associated with poor tumour differentiation.
Allott EH, Lysaght J, Cathcart MC, Donohoe CL, Cummins R, McGarrigle SA, Kay E, Reynolds JV, Pidgeon GP. Allott EH, et al. Mol Carcinog. 2013 Feb;52(2):144-54. doi: 10.1002/mc.21840. Epub 2011 Nov 28. Mol Carcinog. 2013. PMID: 22121096 - Periprostatic adipose tissue and prostate cancer progression: new insights into the tumor microenvironment.
Toren P, Venkateswaran V. Toren P, et al. Clin Genitourin Cancer. 2014 Feb;12(1):21-6. doi: 10.1016/j.clgc.2013.07.013. Epub 2013 Oct 24. Clin Genitourin Cancer. 2014. PMID: 24269373 Review. No abstract available. - Interplay between Prostate Cancer and Adipose Microenvironment: A Complex and Flexible Scenario.
Cancel M, Pouillot W, Mahéo K, Fontaine A, Crottès D, Fromont G. Cancel M, et al. Int J Mol Sci. 2022 Sep 15;23(18):10762. doi: 10.3390/ijms231810762. Int J Mol Sci. 2022. PMID: 36142673 Free PMC article. Review.
Cited by
- The Role of Periprostatic Adipose Tissue on Prostate Function in Vascular-Related Disorders.
Passos GR, Ghezzi AC, Antunes E, de Oliveira MG, Mónica FZ. Passos GR, et al. Front Pharmacol. 2021 Feb 12;12:626155. doi: 10.3389/fphar.2021.626155. eCollection 2021. Front Pharmacol. 2021. PMID: 33643052 Free PMC article. Review. - Magnetic resonance imaging-based radiomics nomogram for the evaluation of therapeutic responses to neoadjuvant chemohormonal therapy in high-risk non-metastatic prostate cancer.
Wu XH, Ruan ZT, Ke ZB, Lin F, Chen JY, Xue YT, Lin B, Chen SH, Chen DN, Zheng QS, Xue XY, Wei Y, Xu N. Wu XH, et al. Cancer Med. 2024 Jul;13(14):e70001. doi: 10.1002/cam4.70001. Cancer Med. 2024. PMID: 39031016 Free PMC article. - Androgen deprivation therapy promotes an obesity-like microenvironment in periprostatic fat.
Mangiola S, Stuchbery R, McCoy P, Chow K, Kurganovs N, Kerger M, Papenfuss A, Hovens CM, Corcoran NM. Mangiola S, et al. Endocr Connect. 2019 May 1;8(5):547-558. doi: 10.1530/EC-19-0029. Endocr Connect. 2019. PMID: 30959474 Free PMC article. - MRI-measured periprostatic to subcutaneous adipose tissue thickness ratio as an independent risk factor in prostate cancer patients undergoing radical prostatectomy.
Jiang S, Li Y, Guo Y, Gong B, Wei C, Liu W, Chen C, Pan F, Song J, He Q, Yang L, Zhou G. Jiang S, et al. Sci Rep. 2024 Sep 8;14(1):20896. doi: 10.1038/s41598-024-71862-w. Sci Rep. 2024. PMID: 39245685 Free PMC article. - Periprostatic Adipose Tissue: A New Perspective for Diagnosing and Treating Prostate Cancer.
Cao H, Wang Y, Zhang D, Liu B, Zhou H, Wang S. Cao H, et al. J Cancer. 2024 Jan 1;15(1):204-217. doi: 10.7150/jca.89750. eCollection 2024. J Cancer. 2024. PMID: 38164282 Free PMC article. Review.
References
- Capitanio U, Suardi N, Briganti A, Gallina A, Abdollah F, Lughezzani G, Salonia A, Freschi M, Montorsi F. Influence of obesity on tumour volume in patients with prostate cancer. BJU Int. 2011;109:678–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous