The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation - PubMed (original) (raw)
. 2012 Aug;22(8):1246-57.
doi: 10.1038/cr.2012.52. Epub 2012 Apr 3.
Cixiong Zhang, Qi Liu, Qinxi Li, Guili Lian, Di Wu, Xuebin Li, Wei Zhang, Yuemao Shen, Zhiyun Ye, Shu-Yong Lin, Sheng-Cai Lin
Affiliations
- PMID: 22473005
- PMCID: PMC3411167
- DOI: 10.1038/cr.2012.52
The Axin/TNKS complex interacts with KIF3A and is required for insulin-stimulated GLUT4 translocation
Hui-Ling Guo et al. Cell Res. 2012 Aug.
Abstract
Insulin-stimulated glucose uptake by the glucose transporter GLUT4 plays a central role in whole-body glucose homeostasis, dysregulation of which leads to type 2 diabetes. However, the molecular components and mechanisms regulating insulin-stimulated glucose uptake remain largely unclear. Here, we demonstrate that Axin interacts with the ADP-ribosylase tankyrase 2 (TNKS2) and the kinesin motor protein KIF3A, forming a ternary complex crucial for GLUT4 translocation in response to insulin. Specific knockdown of the individual components of the complex attenuated insulin-stimulated GLUT4 translocation to the plasma membrane. Importantly, TNKS2(-/-) mice exhibit reduced insulin sensitivity and higher blood glucose levels when re-fed after fasting. Mechanistically, we demonstrate that in the absence of insulin, Axin, TNKS and KIF3A are co-localized with GLUT4 on the trans-Golgi network. Insulin treatment suppresses the ADP-ribosylase activity of TNKS, leading to a reduction in ADP ribosylation and ubiquitination of both Axin and TNKS, and a concurrent stabilization of the complex. Inhibition of Akt, the major effector kinase of insulin signaling, abrogates the insulin-mediated complex stabilization. We have thus elucidated a new protein complex that is directly associated with the motor protein kinesin in insulin-stimulated GLUT4 translocation.
Figures
Figure 1
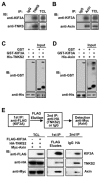
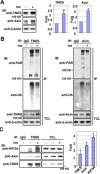
Axin forms a ternary complex with TNKS and KIF3A. (A) TNKS interacts with KIF3A. Lysates of 3T3-L1 adipocytes were subjected to IP with control IgG or rabbit anti-TNKS polyclonal antibody for endogenous TNKS. The immunoprecipitates along with total cell lysates were analyzed by immunoblotting (IB) separately with anti-KIF3A and anti-TNKS antibodies. (B) Axin binds KIF3A. The experiment was performed in the same way as in A, except that anti-Axin antibody was used for IP. (C, D) Interactions between TNKS2 and KIF3A (C) and between Axin and KIF3A (D) were examined by in vitro pull-down assays. His-tagged TNKS2 or Axin was incubated with GST or GST-tagged KIF3A for 4 h. The samples were then incubated with Ni-NTA beads to pull down His-tagged TNKS2 or Axin, followed by immunoblotting with anti-His or anti-GST antibody. (E) Axin forms a ternary complex with TNKS2 and KIF3A. HA-TNKS2 and Myc-Axin were cotransfected with or without FLAG-KIF3A into 293T as indicated beneath TCL. A two-step co-IP was performed as outlined. The FLAG antibody co-precipitated HA-TNKS2 and Myc-Axin only from the cells cotransfected with FLAG-KIF3A.
Figure 2
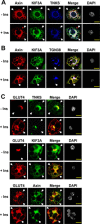
Axin, TNKS and KIF3A are co-localized and highly enriched in the perinuclear region. Differentiated 3T3-L1 adipocytes (d 8) were incubated with or without 200 nM insulin for 30 min after starvation in low-glucose DMEM for 4 h. (A) Cellular Axin, KIF3A and TNKS are co-localized in the perinuclear region. The nuclei were indicated by DAPI staining (gray). Images were acquired with a confocal laser-scanning microscope. After superimposition of images, white color indicates co-localization of Axin (red), KIF3A (green), and TNKS (blue). Arrow heads point to the PM. (B) Axin and KIF3A are partially co-localized with the trans Golgi network marker TGN38. (C) TNKS, KIF3A and Axin have co-localization with GLUT4. Scale bars represent 15 μm.
Figure 3
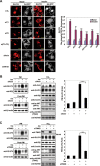
Knockdown of Axin, TNKS or KIF3A impaired GLUT4 translocation. 3T3-L1 cells were induced to differentiate into adipocytes and on day 6 were infected with adenovirus vector (pAdEasy-1) expressing different siRNAs as indicated. (A) Knockdown of Axin, TNKS or KIF3A blocks insulin-dependent GLUT4 translocation. After 48 h of infection with the indicated siRNA-delivering adenovirus, adipocytes were serum starved for 4 h, followed by incubation with or without 200 nM insulin for 30 min. Cells were then fixed and analyzed by immunofluorescence staining using the GLUT4 antibody and a Texas Red-coupled secondary antibody. Arrow heads point to the PM. The outlines of adipocytes (gray) were displayed by GFP co-expressed from adenovirus. Scale bars represent 20 μm. The siRNA effects on GLUT4 translocation were re-assessed by Rim intensity/TOTAL fluorescence (Right), which were collected and analyzed using Leica TCS SP2 confocal laser scanning software. The results show that knockdown of each component of the Axin complex attenuates insulin-stimulated GLUT4 translocation to the cell membrane (right, **P < 0.01; ***P < 0.001). (B, C) Knockdown of Axin (B) or TNKS (double knockdown of TNKS1 and TNKS2) (C) attenuates insulin-stimulated GLUT4 translocation to PM. Adipocytes were infected with adenovirus on day 6 and 48 h later PM fractions were prepared as described in Materials and Methods after insulin (200 nM for 30 min) stimulation. Equal amounts (15 μg) of protein of the PM fractions (membrane pellet) and the Post-PM (representing cell supernatant) fractions, along with the total cell lysates (TCL), were subjected to immunoblotting using antibodies indicated. The immunoblots of PM GLUT4 were quantified by densitometry on the right. (Data are mean ± SEM of three independent experiments. ***P < 0.001.)
Figure 4
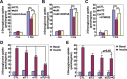
Knockdown of components of the Axin complex attenuated insulin-stimulated glucose uptake. Single knockdown of Axin (siA) (A), KIF3A (siK) (B) or TNKS2 (siT2, **P < 0.01; ***P < 0.001) (C), and double knockdown of TNKS1 and TNKS2 (D) or KIF3A and TNKS2 (E) were carried out, respectively. 3T3-L1 cells were induced to differentiate into adipocytes and on day 6 were infected with adenovirus vector (pAdEasy-1) expressing different siRNAs as indicated. Experiments were performed 48 h after infection according to the protocol described in Materials and Methods.
Figure 5
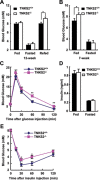
TNKS2 is required in insulin-stimulated glucose disposal. (A) Analysis of blood glucose concentrations in normal and mutant adult mice (12-week males). Mice (TNKS2+/+, n = 6; _TNKS2_−/−, n = 5) were fasted for 16 h, and re-fed for 1 h. Blood glucose levels in the fasted mice and re-fed mice were separately determined. (B) Blood glucose levels of young (7-week) males (TNKS2+/+, n = 7; _TNKS2_−/−, n = 8) were determined, showing a significant difference between the WT and mutant mice (*P < 0.05). (C) Glucose tolerance test. Animals were fasted for 16 h. D-Glucose (2 g/kg) was administrated into mice by intraperitoneal injection, and blood glucose concentrations were determined at the indicated times (12-week male mice; TNKS2+/+, n = 6, 24-27 g; _TNKS2_−/−, n = 5, 20-24 g; *P < 0.05, **P < 0.01). (D) WT (n = 13) and mutant mice (n = 12) were fed or fasted overnight, and their insulin levels were determined. (E) Insulin tolerance test. 1 U/kg human insulin was administered into 12-week mice (TNKS2+/+, n = 6, 24-27 g; _TNKS2_−/−, n = 5, 20-24 g) by intraperitoneal injection and blood glucose levels were determined at the indicated times.
Figure 6
Insulin stabilizes the Axin/TNKS/KIF3A complex by suppressing TNKS PARsylation activity. (A) Insulin treatment increases protein levels of TNKS and Axin. Differentiated 3T3-L1 adipocytes were treated with or without 200 nM insulin for 30 min after 4 h serum deprivation, and were harvested with SDS sample buffer for western blotting with antibodies for endogenous TNKS and Axin. The bar graphs show basal (lane 1) and insulin-stimulated (lane 2) TNKS and Axin protein levels (normalized to lane 1) determined from immunoblotting. (B) PARsylation and ubiquitination of TNKS (left panel) and Axin (right panel) were decreased after insulin treatment. Adipocytes were serum starved for 4 h, and then incubated with DMEM supplemented with or without 200 nM insulin for 30 min in the presence of 20 μM MG132. The endogenous TNKS proteins were immunoprecipitated with anti-TNKS, followed by analysis of their levels of ADP-ribosylation and ubiquitination using anti-poly-ADP-ribose (PAR) and anti-ubiquitin antibodies, respectively. (C) Insulin treatment elevates the abundance of the Axin/TNKS/KIF3A complex. Adipocytes were serum-depleted for 4 h, then added with or without insulin to a final concentration of 200 nM, and incubated for 30 min before lysis. The endogenous proteins were immunoprecipitated with anti-TNKS antibody, followed by immunoblotting with individual antibodies to determine levels of each protein in the complex, quantified by densitometry after normalizing to their respective levels from the unstimulated cells.
Figure 7
Akt dependence of insulin-induced accumulation of the Axin/TNKS/KIF3A complex. (A) Inhibition of Akt attenuates insulin-induced complex formation. 3T3-L1 cells were serum starved for 4 h and then incubated with 1.8 μM Akt inhibitor VIII (inh) and 200 nM insulin (ins) for 30 min, followed by IP with anti-TNKS antibody. The bar graphs (on the right) show fold increases of indicated protein on lanes 2-5 (normalized to lane 2). The precipitated TNKS and the co-precipitated proteins were analyzed by immunoblotting using specific antibodies as indicated. (B) Inhibition of Akt antagonizes the effect of insulin on TNKS PARsylation and ubiquitination. Insulin treatment decreases PARsylation and ubiquitination of TNKS (lane 3 compared to lane 2), which was abrogated by the Akt inhibitor. (C) A simplified model showing that the Axin/TNKS complex is connected to GLUT4 vesicles trafficking by KIF3A. Axin, TNKS and KIF3A form a ternary complex that is associated with the TGN. In the absence of insulin, TNKS and Axin are degraded after poly-ADP-ribosylation and ubiquitination. Insulin treatment prevents the PARsylation of TNKS and Axin in an Akt-dependent manner to increase the abundance of the Axin/TNKS/KIF3A complex, in that Axin acts to enhance the complex. It has been shown that insulin-activated Akt phosphorylates AS160 that is a GTPase-activating protein for Rabs; upon phosphorylation, AS160 can no longer exert its inhibitory effect on Rabs, ultimately allowing GSV to translocate to the cell surface,,,,,.
Similar articles
- Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes.
Habtemichael EN, Li DT, Alcázar-Román A, Westergaard XO, Li M, Petersen MC, Li H, DeVries SG, Li E, Julca-Zevallos O, Wolenski JS, Bogan JS. Habtemichael EN, et al. J Biol Chem. 2018 Jul 6;293(27):10466-10486. doi: 10.1074/jbc.RA118.003021. Epub 2018 May 17. J Biol Chem. 2018. PMID: 29773651 Free PMC article. - Insulin-stimulated exocytosis of GLUT4 is enhanced by IRAP and its partner tankyrase.
Yeh TY, Sbodio JI, Tsun ZY, Luo B, Chi NW. Yeh TY, et al. Biochem J. 2007 Mar 1;402(2):279-90. doi: 10.1042/BJ20060793. Biochem J. 2007. PMID: 17059388 Free PMC article. - The Poly(ADP-ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-ribosylation of Axin and APC2.
Croy HE, Fuller CN, Giannotti J, Robinson P, Foley AVA, Yamulla RJ, Cosgriff S, Greaves BD, von Kleeck RA, An HH, Powers CM, Tran JK, Tocker AM, Jacob KD, Davis BK, Roberts DM. Croy HE, et al. J Biol Chem. 2016 Jun 10;291(24):12747-12760. doi: 10.1074/jbc.M115.705442. Epub 2016 Apr 11. J Biol Chem. 2016. PMID: 27068743 Free PMC article. - Fluorescence microscopy-based quantitation of GLUT4 translocation.
Heckmann M, Klanert G, Sandner G, Lanzerstorfer P, Auer M, Weghuber J. Heckmann M, et al. Methods Appl Fluoresc. 2022 Jan 21;10(2). doi: 10.1088/2050-6120/ac4998. Methods Appl Fluoresc. 2022. PMID: 35008072 Review. - The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets.
Leney SE, Tavaré JM. Leney SE, et al. J Endocrinol. 2009 Oct;203(1):1-18. doi: 10.1677/JOE-09-0037. Epub 2009 Apr 23. J Endocrinol. 2009. PMID: 19389739 Review.
Cited by
- Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes.
Habtemichael EN, Li DT, Alcázar-Román A, Westergaard XO, Li M, Petersen MC, Li H, DeVries SG, Li E, Julca-Zevallos O, Wolenski JS, Bogan JS. Habtemichael EN, et al. J Biol Chem. 2018 Jul 6;293(27):10466-10486. doi: 10.1074/jbc.RA118.003021. Epub 2018 May 17. J Biol Chem. 2018. PMID: 29773651 Free PMC article. - Poly(ADP-ribose) polymerase enzymes and the maintenance of genome integrity.
Eisemann T, Pascal JM. Eisemann T, et al. Cell Mol Life Sci. 2020 Jan;77(1):19-33. doi: 10.1007/s00018-019-03366-0. Epub 2019 Nov 21. Cell Mol Life Sci. 2020. PMID: 31754726 Free PMC article. Review. - The PARsylation activity of tankyrase in adipose tissue modulates systemic glucose metabolism in mice.
Zhong L, Ding Y, Bandyopadhyay G, Waaler J, Börgeson E, Smith S, Zhang M, Phillips SA, Mahooti S, Mahata SK, Shao J, Krauss S, Chi NW. Zhong L, et al. Diabetologia. 2016 Mar;59(3):582-91. doi: 10.1007/s00125-015-3815-1. Epub 2015 Dec 2. Diabetologia. 2016. PMID: 26631215 - ADP-ribosylation signalling and human disease.
Palazzo L, Mikolčević P, Mikoč A, Ahel I. Palazzo L, et al. Open Biol. 2019 Apr 26;9(4):190041. doi: 10.1098/rsob.190041. Open Biol. 2019. PMID: 30991935 Free PMC article. Review. - Structural insights into SAM domain-mediated tankyrase oligomerization.
DaRosa PA, Ovchinnikov S, Xu W, Klevit RE. DaRosa PA, et al. Protein Sci. 2016 Sep;25(9):1744-52. doi: 10.1002/pro.2968. Epub 2016 Jul 4. Protein Sci. 2016. PMID: 27328430 Free PMC article.
References
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. - PubMed
- Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34:481–487. - PubMed
- Leney SE, Tavare JM. The molecular basis of insulin-stimulated glucose uptake: signalling, trafficking and potential drug targets. J Endocrinol. 2009;203:1–18. - PubMed
- Martin S, Haruta T, Morris A, Klippel A, Williams L, Olefsky J. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical