2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis - PubMed (original) (raw)
Review
doi: 10.1002/acr.21641.
Daniel E Furst, Aseem Bharat, Jeffrey R Curtis, Arthur F Kavanaugh, Joel M Kremer, Larry W Moreland, James O'Dell, Kevin L Winthrop, Timothy Beukelman, S Louis Bridges Jr, W Winn Chatham, Harold E Paulus, Maria Suarez-Almazor, Claire Bombardier, Maxime Dougados, Dinesh Khanna, Charles M King, Amye L Leong, Eric L Matteson, John T Schousboe, Eileen Moynihan, Karen S Kolba, Archana Jain, Elizabeth R Volkmann, Harsh Agrawal, Sangmee Bae, Amy S Mudano, Nivedita M Patkar, Kenneth G Saag
Affiliations
- PMID: 22473917
- PMCID: PMC4081542
- DOI: 10.1002/acr.21641
Review
2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis
Jasvinder A Singh et al. Arthritis Care Res (Hoboken). 2012 May.
No abstract available
Conflict of interest statement
Financial Conflict: Forms submitted as required
Figures
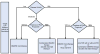
Figure 1. 2012 American College of Rheumatology Recommendations Update for Treatment of Early Rheumatoid Arthritis, Defined as Disease Duration < 6 Months
1Definitions of disease activity are discussed in Tables 2 and 3 and Appendix 4 and were categorized as low, moderate or high 2Patients were categorized based on presence or absence of one or more of the following poor prognostic features: functional limitation (e.g., health assessment questionnaire (HAQ) score or similar valid tools); extra-articular disease (e.g., presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome); positive rheumatoid factor (RF) or anti-cyclic citrullinated peptide (CCP) antibodies (–37); bony erosions by radiograph (38) HCQ, Hydroxychloroquine; MTX, methotrexate; DMARD, Disease-Modifying Anti-Rheumatic Drug- includes hydroxychloroquine, leflunomide, methotrexate, minocycline and sulfasalazine; HAQ-DI, Health Assessment Questionnaire Disability Index; TNF, Tumor-necrosis Factor For the level of evidence supporting each recommendation, please see Appendix 7.
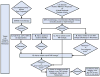
Figure 2. 2012 American College of Rheumatology Recommendations Update for Treatment of Established Rheumatoid Arthritis (Disease Duration≥ 6 months or Meeting 1987 ACR Classification Criteria)
* Reassess after 3 months and proceed escalating therapy if Moderate or High Disease Activity in all instances except after treatment with non-TNF biologic (rectangle D), where reassessment is recommended at 6-months due to a longer anticipated time for peak effect 1Definitions of disease activity are discussed in Tables 2 and 3 and Appendix 4 and were categorized as low, moderate or high 2Features of poor prognosis included the presence of one or more of the following: functional limitation (e.g., health assessment questionnaire (HAQ) score or similar valid tools); extra-articular disease (e.g., presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome); positive rheumatoid factor (RF) or anti-cyclic citrullinated peptide (CCP) antibodies (–37); bony erosions by radiograph (38) 3Combination DMARD therapy with two DMARDs, which is most commonly methotrexate-based with few exceptions (for example, methotrexate + hydroxychloroquine, methotrexate + leflunomide, methotrexate + sulfasalazine, sulfasalazine + hydroxychloroquine) and triple therapy (methotrexate + hydroxychloroquine + sulfasalazine). 4Leflunomide can be added in patients with low disease activity after 3–6 months of minocycline, hydroxychloroquine, methotrexate or sulfasalazine 5If after 3 months of intensified DMARD combination-therapy or after a second DMARD has failed, the option is to add or switch to an anti-TNF biologic. 6Reassessment after treatment with non-TNF biologic is recommended at 6-months due to anticipation that a longer time to peak effect is needed for non-TNF compared to anti-TNF biologics. 7Any adverse event was defined as per the U.S. FDA as any undesirable experience associated with the use of a medical product in a patient. Serious adverse events were defined per the U.S. FDA (see below); all other adverse events were considered non-serious adverse events. The FDA definition of serious adverse event includes death, life-threatening event, initial or prolonged hospitalization, disability, congenital anomaly or an adverse event requiring intervention to prevent permanent impairment or damage. For the level of evidence supporting each recommendation, please see Appendix 7. MTX, methotrexate; LEF, leflunomide; HCQ, Hydroxychloroquine; TNF, Tumor-necrosis factor DMARD, Disease-Modifying Anti-Rheumatic Drug include hydroxychloroquine, leflunomide, methotrexate, minocycline and sulfasalazine (therapies are listed alphabetically; azathioprine and cyclosporine were considered but not included) DMARD monotherapy refers to treatment in most instances with hydroxychloroquine, leflunomide, methotrexate or sulfasalazine; in few instances, where appropriate, minocycline may also be used Anti-TNF biologics include adalimumab, certolizumab, etanercept, infliximab, golimumab Non-TNF biologics include abatacept, rituximab or tocilizumab (therapies are listed alphabetically)
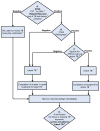
Figure 3. 2012 American College of Rheumatology Recommendations Update for Tuberculosis Screening with Biologic Use
1Anergy panel testing is not recommended 2IGRA is preferred if patient has a history of Bacillus-Calmette-Guérin (BCG) vaccination 3Risk factors for TB exposure are defined based on a publication from the US Centers for Disease Control and Prevention (CDC) as: close contacts of persons known or suspected to have active tuberculosis; foreign-born persons from areas that have a high incidence of active tuberculosis (e.g., Africa, Asia, Eastern Europe, Latin America, and Russia); persons who visit areas with a high prevalence of active tuberculosis, especially if visits are frequent or prolonged; residents and employees of congregate settings whose clients are at increased risk for active tuberculosis (e.g., correctional facilities, long-term care facilities, and homeless shelters); health-care workers who serve clients who are at increased risk for active tuberculosis; populations defined locally as having an increased incidence of latent M. tuberculosis infection or active tuberculosis, possibly including medically underserved, low-income populations, or persons who abuse drugs or alcohol; and infants, children, and adolescents exposed to adults who are at increased risk for latent M. tuberculosis infection or active tuberculosis. (16) 4If patient is immunosuppressed and false negative results more likely, consider repeating screening test(s) with TST or IGRA. 5Chest radiograph may also be considered when clinically indicated in patients with risk factors, even with a negative repeat TST or IGRA. 6Obtain respiratory (e.g., sputum, bronchoalveolar lavage) or other samples as clinically appropriate for AFB smear and culture. Consider referral to TB specialist for further work-up and treatment. 7In a patient diagnosed with latent or active TB, consider referral to a specialist for the recommended treatment. 8Patients who test positive for TST or IGRA at baseline often remain positive for these tests even after successful treatment of TB. These patients need monitoring for clinical signs and symptoms of recurrent TB disease, since repeating tests will not allow help in diagnosis of recurrent TB. The level of evidence supporting each recommendation for TB reactivation was “C”, except for initiation of biologics in patients being treated for latent TB infection (LTBI), where the Level of Evidence was “B”) TST, Tuberculin Skin Test; IGRA, Interferon-gamma release assay; AFB, Acid fast bacilli
Comment in
- Updated recommendations for the treatment of rheumatoid arthritis: another step on a long road.
Daikh DI, St Clair EW. Daikh DI, et al. Arthritis Care Res (Hoboken). 2012 May;64(5):648-51. doi: 10.1002/acr.21659. Arthritis Care Res (Hoboken). 2012. PMID: 22473919 Free PMC article. No abstract available. - American College of Rheumatology treatment guidelines continue to omit guidance on glucocorticoids: comment on the article by Singh et al.
Boers M, Kirwan JR, Bijlsma JW. Boers M, et al. Arthritis Care Res (Hoboken). 2012 Oct;64(10):1622. doi: 10.1002/acr.21770. Arthritis Care Res (Hoboken). 2012. PMID: 22740377 No abstract available. - Reply: To PMID 22473917.
Singh JA, Saag KG, Furst D. Singh JA, et al. Arthritis Care Res (Hoboken). 2013 May;65(5):832-3. doi: 10.1002/acr.21872. Arthritis Care Res (Hoboken). 2013. PMID: 23097242 No abstract available. - Level of the indication of biologic agents in the 2012 American College of Rheumatology recommendations for the treatment of rheumatoid arthritis: comment on the article by Singh et al.
Graudal N, Jürgens G. Graudal N, et al. Arthritis Care Res (Hoboken). 2013 May;65(5):832. doi: 10.1002/acr.21873. Arthritis Care Res (Hoboken). 2013. PMID: 23097263 No abstract available.
Similar articles
- Level of the indication of biologic agents in the 2012 American College of Rheumatology recommendations for the treatment of rheumatoid arthritis: comment on the article by Singh et al.
Graudal N, Jürgens G. Graudal N, et al. Arthritis Care Res (Hoboken). 2013 May;65(5):832. doi: 10.1002/acr.21873. Arthritis Care Res (Hoboken). 2013. PMID: 23097263 No abstract available. - Current therapeutic agents and treatment paradigms for the management of rheumatoid arthritis.
Gibofsky A. Gibofsky A. Am J Manag Care. 2014 May;20(7 Suppl):S136-44. Am J Manag Care. 2014. PMID: 25180622 Review. - Recommendations for the use of biologic therapy in rheumatoid arthritis: update from the Italian Society for Rheumatology I. Efficacy.
Caporali R, Conti F, Alivernini S, Atzeni F, Seriolo B, Cutolo M, Valesini G, Ferraccioli G, Sarzi-Puttini P, Salvarani C, Guiducci S, Zampogna G, Gremese E, Pipitone N, Scrivo R, Bugatti S, Montecucco C, Matucci-Cerinic M; Italian Society for Rheumatology. Caporali R, et al. Clin Exp Rheumatol. 2011 May-Jun;29(3 Suppl 66):S7-14. Epub 2011 Jul 26. Clin Exp Rheumatol. 2011. PMID: 21906423 Review.
Cited by
- Personalizing guidelines for diabetes management: twilight or dawn of the expert?
Paschou SA, Leslie RD. Paschou SA, et al. BMC Med. 2013 Jul 10;11:161. doi: 10.1186/1741-7015-11-161. BMC Med. 2013. PMID: 23841986 Free PMC article. Review. - Cancer Recurrence Following Immune-Suppressive Therapies in Patients With Immune-Mediated Diseases: A Systematic Review and Meta-analysis.
Shelton E, Laharie D, Scott FI, Mamtani R, Lewis JD, Colombel JF, Ananthakrishnan AN. Shelton E, et al. Gastroenterology. 2016 Jul;151(1):97-109.e4. doi: 10.1053/j.gastro.2016.03.037. Epub 2016 Apr 1. Gastroenterology. 2016. PMID: 27039969 Free PMC article. Review. - 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis.
Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, Kremer J, Nakamura MC, Russell LA, Singh JA, Smith BJ, Sparks JA, Venkatachalam S, Weinblatt ME, Al-Gibbawi M, Baker JF, Barbour KE, Barton JL, Cappelli L, Chamseddine F, George M, Johnson SR, Kahale L, Karam BS, Khamis AM, Navarro-Millán I, Mirza R, Schwab P, Singh N, Turgunbaev M, Turner AS, Yaacoub S, Akl EA. Fraenkel L, et al. Arthritis Care Res (Hoboken). 2021 Jul;73(7):924-939. doi: 10.1002/acr.24596. Epub 2021 Jun 8. Arthritis Care Res (Hoboken). 2021. PMID: 34101387 Free PMC article. - Tumour necrosis factor inhibitors versus combination intensive therapy with conventional disease modifying anti-rheumatic drugs in established rheumatoid arthritis: TACIT non-inferiority randomised controlled trial.
Scott DL, Ibrahim F, Farewell V, O'Keeffe AG, Walker D, Kelly C, Birrell F, Chakravarty K, Maddison P, Heslin M, Patel A, Kingsley GH. Scott DL, et al. BMJ. 2015 Mar 13;350:h1046. doi: 10.1136/bmj.h1046. BMJ. 2015. PMID: 25769495 Free PMC article. Clinical Trial. - The effectiveness of tofacitinib, a novel Janus kinase inhibitor, in the treatment of rheumatoid arthritis: a systematic review and meta-analysis.
Kawalec P, Mikrut A, Wiśniewska N, Pilc A. Kawalec P, et al. Clin Rheumatol. 2013 Oct;32(10):1415-24. doi: 10.1007/s10067-013-2329-9. Epub 2013 Jul 23. Clin Rheumatol. 2013. PMID: 23877486 Free PMC article. Review.
References
- Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–84. Epub 2008/06/03. - PubMed
- McGory ML, Shekelle PG, Rubenstein LZ, Fink A, Ko CY. Developing quality indicators for elderly patients undergoing abdominal operations. J Am Coll Surg. 2005;201(6):870–83. Epub 2005/11/29. - PubMed
- Shekelle P. The appropriateness method. Med Decis Making. 2004;24(2):228–31. Epub 2004/04/20. - PubMed
- Shekelle PG, Park RE, Kahan JP, Leape LL, Kamberg CJ, Bernstein SJ. Sensitivity and specificity of the RAND/UCLA Appropriateness Method to identify the overuse and underuse of coronary revascularization and hysterectomy. J Clin Epidemiol. 2001;54(10):1004–10. Epub 2001/09/29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical