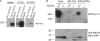Interaction of voltage-gated sodium channel Nav1.6 (SCN8A) with microtubule-associated protein Map1b - PubMed (original) (raw)
Interaction of voltage-gated sodium channel Nav1.6 (SCN8A) with microtubule-associated protein Map1b
Janelle E O'Brien et al. J Biol Chem. 2012.
Abstract
The mechanism by which voltage-gated sodium channels are trafficked to the surface of neurons is not well understood. Our previous work implicated the cytoplasmic N terminus of the sodium channel Na(v)1.6 in this process. We report that the N terminus plus the first transmembrane segment (residues 1-153) is sufficient to direct a reporter to the cell surface. To identify proteins that interact with the 117-residue N-terminal domain, we carried out a yeast two-hybrid screen of a mouse brain cDNA library. Three clones containing overlapping portions of the light chain of microtubule-associated protein Map1b (Mtap1b) were recovered from the screen. Interaction between endogenous Na(v)1.6 channels and Map1b in mouse brain was confirmed by co-immunoprecipitation. Map1b did not interact with the N terminus of the related channel Na(v)1.1. Alanine-scanning mutagenesis of the Na(v)1.6 N terminus demonstrated that residues 77-80 (VAVP) contribute to interaction with Map1b. Co-expression of Na(v)1.6 with Map1b in neuronal cell line ND7/23 resulted in a 50% increase in current density, demonstrating a functional role for this interaction. Mutation of the Map1b binding site of Na(v)1.6 prevented generation of sodium current in transfected cells. The data indicate that Map1b facilitates trafficking of Na(v)1.6 to the neuronal cell surface.
Figures
FIGURE 1.
N terminus and first transmembrane segment of Nav1.6 are sufficient to direct a reporter protein to the cell surface. Confocal images of HEK293 cells transfected with the reporter constructs and probed with anti-CD4 antibodies are shown. Green, anti-CD74; blue, DAPI. A, extracellular domain of CD74 lacking membrane-targeting N terminus. B, N terminus and first transmembrane segment of Nav1.6 fused to the extracellular domain of CD74. C, ataxia3 mutation S21P not disrupting surface localization.
FIGURE 2.
Yeast two-hybrid screen using the N terminus of Nav1.6 as bait identified Map1b as an interactant. A, schematic of Nav1.6 channel. Residues 1–117 were used as the bait for the yeast two-hybrid screen. B, location of the three independent clones of Map1b identified in the screen. Residues 1924–2426 were cloned into the prey vector for the directed yeast two-hybrid and into pCMV-myc for mammalian cell culture experiments. C, yeast two-hybrid experiments confirming interaction between N terminus of Nav1.6 and Map1b.
FIGURE 3.
Interaction of Nav1.6 with Map1b. A, HEK293 cells co-transfected with Nav1.6-CD74 and myc-Map1b. Map1b light chain co-immunoprecipitates with Nav1.6-CD74 fusion protein from co-transfected cells. B, voltage-gated sodium channels and Map1b light chain co-immunoprecipitated from brain membrane fractions from wild-type (+/+) but not Nav1.6 null (−/−) mice. Lanes contain 50 μg of protein (brain) or 100 μg of protein (immunoprecipitate).
FIGURE 4.
Map1b does not interact with the N terminus of Nav1.1. Co-transformed yeast were plated on selective −Leu/−Trp/−His/−Ade medium. All transformed yeast grew on −Leu/−Trp medium, which selects for presence of the constructs (data not shown). A, yeast two-hybrid interaction of Map1b with the N terminus of Nav1.6 (8A) and Nav1.1 (1A). B, yeast two-hybrid interaction of Map1b with the hybrid constructs 1A(1–54)/8A(55–117) and 8A(1–54)/1A(55–117). C, residues 55–117 of Scn8a and Scn1a differing at 16 of 64 residues.
FIGURE 5.
Localization of Map1b interaction site within N terminus of Nav1.6. A, 15 deletion constructs assayed for interaction with Map1b using the yeast-2-hybrid assay. C-terminal deletion to residue 80 or beyond prevented interaction with Map1b (asterisks). The internal residue 38–90 was sufficient for interaction. B, alanine-scanning constructs spanning the region between residues 73 and 90. Mutation of residues 77–80 prevented interaction with Map1b. +, growth on stringent selection plates; −, no growth.
FIGURE 6.
Co-expression of Map1b increases Nav1.6 peak current density in ND7/23 cells transfected with Nav1.6R. A and B, representative sodium currents were recorded from ND7/23 cells transiently co-transfected with Nav1.6R, EGFP, and vector (n = 23) (A) or Map1b (n = 20) (B). Cells were held at −120 mV, and sodium currents were elicited by a series of step depolarizations from −80 to +40 mV in 5-mV increments. C, co-expression of Map1b significantly increases current density of Nav1.6 in ND7/23 cells (**, p < 0.01). D, Map1b does not alter activation or steady-state fast inactivation of Nav1.6.
FIGURE 7.
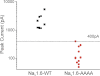
Mutation of the Map1b binding site of Nav1.6R prevents generation of sodium current in transfected ND7/23 cells. Cells were transfected with Map1b and wild-type Nav1.6R or the mutant Nav1.6R-V77A/V79A/P80A and analyzed by whole cell voltage clamp electrophysiology as described in Fig. 6. The threshold of 400 pA for peak current represents the minimum required to construct an unambiguous I-V curve. Peak current amplitude for wild-type channels was >1000 pA (n = 9), whereas cells transfected with the Nav1.6R mutant channel (n = 14) did not produce current above threshold.
FIGURE 8.
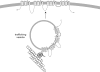
Potential role of interaction between Nav1.6 and Map1b in trafficking of Nav1.6 to the cell membrane. According to this model, interaction with Map1b facilitates trafficking of Nav1.6 along axonal microtubules to the AIS or node of Ranvier. At these sites, the channel is stabilized by ankyrin G and other molecules. M, Map1b light chain.
Similar articles
- The MAP1B Binding Domain of Nav1.6 Is Required for Stable Expression at the Axon Initial Segment.
Solé L, Wagnon JL, Akin EJ, Meisler MH, Tamkun MM. Solé L, et al. J Neurosci. 2019 May 29;39(22):4238-4251. doi: 10.1523/JNEUROSCI.2771-18.2019. Epub 2019 Mar 26. J Neurosci. 2019. PMID: 30914445 Free PMC article. - The ataxia3 mutation in the N-terminal cytoplasmic domain of sodium channel Na(v)1.6 disrupts intracellular trafficking.
Sharkey LM, Cheng X, Drews V, Buchner DA, Jones JM, Justice MJ, Waxman SG, Dib-Hajj SD, Meisler MH. Sharkey LM, et al. J Neurosci. 2009 Mar 4;29(9):2733-41. doi: 10.1523/JNEUROSCI.6026-08.2009. J Neurosci. 2009. PMID: 19261867 Free PMC article. - Molecular determinants for modulation of persistent sodium current by G-protein betagamma subunits.
Mantegazza M, Yu FH, Powell AJ, Clare JJ, Catterall WA, Scheuer T. Mantegazza M, et al. J Neurosci. 2005 Mar 30;25(13):3341-9. doi: 10.1523/JNEUROSCI.0104-05.2005. J Neurosci. 2005. PMID: 15800189 Free PMC article. - Role of the amino and carboxy termini in isoform-specific sodium channel variation.
Lee A, Goldin AL. Lee A, et al. J Physiol. 2008 Aug 15;586(16):3917-26. doi: 10.1113/jphysiol.2008.156299. Epub 2008 Jun 19. J Physiol. 2008. PMID: 18565993 Free PMC article. - Mutations of voltage-gated sodium channels in movement disorders and epilepsy.
Meisler MH, Kearney JA, Sprunger LK, MacDonald BT, Buchner DA, Escayg A. Meisler MH, et al. Novartis Found Symp. 2002;241:72-81; discussion 82-6, 226-32. Novartis Found Symp. 2002. PMID: 11771652 Review.
Cited by
- Immortalized Dorsal Root Ganglion Neuron Cell Lines.
Haberberger RV, Barry C, Matusica D. Haberberger RV, et al. Front Cell Neurosci. 2020 Jun 19;14:184. doi: 10.3389/fncel.2020.00184. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32636736 Free PMC article. Review. - PRRT2 controls neuronal excitability by negatively modulating Na+ channel 1.2/1.6 activity.
Fruscione F, Valente P, Sterlini B, Romei A, Baldassari S, Fadda M, Prestigio C, Giansante G, Sartorelli J, Rossi P, Rubio A, Gambardella A, Nieus T, Broccoli V, Fassio A, Baldelli P, Corradi A, Zara F, Benfenati F. Fruscione F, et al. Brain. 2018 Apr 1;141(4):1000-1016. doi: 10.1093/brain/awy051. Brain. 2018. PMID: 29554219 Free PMC article. - Direct interaction and functional coupling between human 5-HT6 receptor and the light chain 1 subunit of the microtubule-associated protein 1B (MAP1B-LC1).
Kim SH, Kim DH, Lee KH, Im SK, Hur EM, Chung KC, Rhim H. Kim SH, et al. PLoS One. 2014 Mar 10;9(3):e91402. doi: 10.1371/journal.pone.0091402. eCollection 2014. PLoS One. 2014. PMID: 24614691 Free PMC article. - The MAP1B Binding Domain of Nav1.6 Is Required for Stable Expression at the Axon Initial Segment.
Solé L, Wagnon JL, Akin EJ, Meisler MH, Tamkun MM. Solé L, et al. J Neurosci. 2019 May 29;39(22):4238-4251. doi: 10.1523/JNEUROSCI.2771-18.2019. Epub 2019 Mar 26. J Neurosci. 2019. PMID: 30914445 Free PMC article. - Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy.
Wagnon JL, Korn MJ, Parent R, Tarpey TA, Jones JM, Hammer MF, Murphy GG, Parent JM, Meisler MH. Wagnon JL, et al. Hum Mol Genet. 2015 Jan 15;24(2):506-15. doi: 10.1093/hmg/ddu470. Epub 2014 Sep 16. Hum Mol Genet. 2015. PMID: 25227913 Free PMC article.
References
- Schaller K. L., Caldwell J. H. (2000) Developmental and regional expression of sodium channel isoform NaCh6 in the rat central nervous system. J. Comp. Neurol. 420, 84–97 - PubMed
- Raman I. M., Sprunger L. K., Meisler M. H., Bean B. P. (1997) Altered subthreshold sodium currents and disrupted firing patterns in Purkinje neurons of Scn8a mutant mice. Neuron 19, 881–891 - PubMed
- Levin S. I., Khaliq Z. M., Aman T. K., Grieco T. M., Kearney J. A., Raman I. M., Meisler M. H. (2006) Impaired motor function in mice with cell-specific knockout of sodium channel Scn8a (Nav1.6) in cerebellar Purkinje neurons and granule cells. J. Neurophysiol. 96, 785–793 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034509/NS/NINDS NIH HHS/United States
- T32 GM007544/GM/NIGMS NIH HHS/United States
- T32GM007544/GM/NIGMS NIH HHS/United States
- R01 NS34509/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases