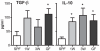Patterns of early gut colonization shape future immune responses of the host - PubMed (original) (raw)
Patterns of early gut colonization shape future immune responses of the host
Camilla Hartmann Friis Hansen et al. PLoS One. 2012.
Abstract
The most important trigger for immune system development is the exposure to microbial components immediately after birth. Moreover, targeted manipulation of the microbiota can be used to change host susceptibility to immune-mediated diseases. Our aim was to analyze how differences in early gut colonization patterns change the composition of the resident microbiota and future immune system reactivity. Germ-free (GF) mice were either inoculated by single oral gavage of caecal content or let colonized by co-housing with specific pathogen-free (SPF) mice at different time points in the postnatal period. The microbiota composition was analyzed by denaturing gradient gel electrophoresis for 16S rRNA gene followed by principal component analysis. Furthermore, immune functions and cytokine concentrations were analyzed using flow cytometry, ELISA or multiplex bead assay. We found that a single oral inoculation of GF mice at three weeks of age permanently changed the gut microbiota composition, which was not possible to achieve at one week of age. Interestingly, the ex-GF mice inoculated at three weeks of age were also the only mice with an increased pro-inflammatory immune response. In contrast, the composition of the gut microbiota of ex-GF mice that were co-housed with SPF mice at different time points was similar to the gut microbiota in the barrier maintained SPF mice. The existence of a short GF postnatal period permanently changed levels of systemic regulatory T cells, NK and NKT cells, and cytokine production. In conclusion, a time window exists that enables the artificial colonization of GF mice by a single oral dose of caecal content, which may modify the future immune phenotype of the host. Moreover, delayed microbial colonization of the gut causes permanent changes in the immune system.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
Figure 1. Gut microbiota comparison of inoculated mice.
Principal Component Analysis (PCA) plot based on DGGE profiles of 16S rRNA gene PCR derived amplicons of feces samples collected from mice in the inoculation experiment at nine weeks of age. Germ-free (GF) mice were inoculated at one week of age (red balls) or at three weeks of age (yellow balls) with a bacteria suspension made from caecal content of specific pathogen-free (SPF) mice from Taconic Farms (blue balls illustrate different aliquots of the suspension). Their gut microbiota was compared with SPF mice raised in our barrier (green balls). ANOVA based on Principal Component (PC) 1 explaining 14.7% of the variance confirmed a significant difference between mice inoculated at three weeks of age and all other mice (p<0.01). No significant differences of PC values were found between ex-GF mice inoculated at three weeks of age and the suspension aliquots used to inoculate these mice with. DGGE: Denaturing Gradient Gel Electrophoresis.
Figure 2. Gut microbiota comparison of co-housed mice.
Principal Component Analysis (PCA) plot based on DGGE profiles of 16S rRNA gene PCR derived amplicons of feces samples collected from mice in the co-housing experiment at nine weeks of age. Germ-free (GF) mice were conventionalized at one week of age (red balls) or at three weeks of age (yellow balls) by co-housing them with specific pathogen-free (SPF) mice (green balls). No differences in gut microbiota composition were detected between the SPF and co-housed ex-GF mice. DGGE: Denaturing Gradient Gel Electrophoresis.
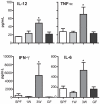
Figure 3. Microbial inoculation at three weeks of age results in pro-inflammatory tuning of the immune system.
Cytokines were measured in mesenteric lymph node (MLN) cell culture supernatants and analyzed by use of FlowCytomix Multiplex Th1/Th2 10plex. MLN were isolated from specific pathogen-free (SPF) mice (SPF), germ-free (GF) mice (GF) and ex-GF mice inoculated with a caecal microbiota suspension at one (1W) or three weeks (3W) of age. The lymphocytes were stimulated for 24 hrs with 1 µg/mL lipopolysaccharide (LPS) from Escherichia coli O26:B6 before the supernatants were used for analysis. Only cytokines with a significant difference among groups are illustrated. Error bars represent the SEM. * represents p<0.05 compared with SPF mice.
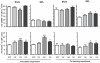
Figure 4. A germ-free postnatal period affects long-term immune-regulatory homeostasis.
Percentages of regulatory T cells (CD4+FoxP3+) and tolerogenic dendritic cells (CD103+CD11c+) in cells isolated from spleen (SPL) and mesenteric lymph node (MLN) were determined by flow cytometry. Specific Pathogen Free mice (SPF), germ-free mice (GF) and ex-GF mice inoculated with a caecal microbiota suspension or co-housed with SPF mice at one (1W) or three weeks (3W) of age are illustrated. Error bars represent the SEM. * represents p<0.05, ** <0.01, *** <0.001 compared with SPF mice.
Figure 5. A germ-free postnatal period increases serum regulatory cytokines.
Analyses of serum cytokines were performed by ELISA. Serum was extracted from Specific Pathogen Free (SPF) mice, germ-free (GF) mice and ex-GF mice co-housed at one (1W) or three weeks (3W) of age with SPF mice. Error bars represent the SEM. * represents p<0.05 compared with SPF mice.
Figure 6. A germ-free postnatal period alters systemic mononuclear cell populations.
Distribution of cell populations in spleen (A) and mesenteric lymph node (B) sampled from mice in the co-housing experiment were measured by flow cytometry. Germ-free (GF) mice were conventionalized at one week of age (1W) or at three weeks of age (3W) by co-housing them with Specific Pathogen Free mice (SPF) raised in our barrier. A group of mice remained germ-free (GF). Percentages of NK cells (CD3−CD49b+), NKT cells (CD3+CD49b+), CD69+ and CD103+ T cells (CD3+CD4+) and IFN-γ producing T cells (CD3+CD4+) are illustrated. Lymphocytes were cultivated for 4 hrs with 50 ng/mL PMA and 750 ng/mL Ionomycin (Sigma-Aldrich) in the presence of BD GolgiStop before cells were harvested and intracellular IFN-γ stained. The mean is illustrated. * represents p<0.05, ** <0.01, *** <0.001 compared with SPF mice. PMA: Phorbol 12-Myristate 13-Acetate.
Similar articles
- Delayed bacterial colonization of the gut alters the host immune response to oral sensitization against cow's milk proteins.
Morin S, Fischer R, Przybylski-Nicaise L, Bernard H, Corthier G, Rabot S, Wal JM, Hazebrouck S. Morin S, et al. Mol Nutr Food Res. 2012 Dec;56(12):1838-47. doi: 10.1002/mnfr.201200412. Epub 2012 Oct 12. Mol Nutr Food Res. 2012. PMID: 23065810 - Short communication: Gut microbial colonization of the mouse colon using faecal transfer was equally effective when comparing rectal inoculation and oral inoculation based on 16S rRNA sequencing.
Lützhøft DO, Sánchez-Alcoholado L, Tougaard P, Junker Mentzel CM, Kot W, Nielsen DS, Hansen AK. Lützhøft DO, et al. Res Vet Sci. 2019 Oct;126:227-232. doi: 10.1016/j.rvsc.2019.09.009. Epub 2019 Sep 17. Res Vet Sci. 2019. PMID: 31627163 - A defined intestinal colonization microbiota for gnotobiotic pigs.
Laycock G, Sait L, Inman C, Lewis M, Smidt H, van Diemen P, Jorgensen F, Stevens M, Bailey M. Laycock G, et al. Vet Immunol Immunopathol. 2012 Oct 15;149(3-4):216-24. doi: 10.1016/j.vetimm.2012.07.004. Epub 2012 Jul 20. Vet Immunol Immunopathol. 2012. PMID: 22868203 - Customizing laboratory mice by modifying gut microbiota and host immunity in an early "window of opportunity".
Hansen CH, Metzdorff SB, Hansen AK. Hansen CH, et al. Gut Microbes. 2013 May-Jun;4(3):241-5. doi: 10.4161/gmic.23999. Epub 2013 Apr 2. Gut Microbes. 2013. PMID: 23549457 Free PMC article. Review. - The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases.
Tlaskalová-Hogenová H, Stěpánková R, Kozáková H, Hudcovic T, Vannucci L, Tučková L, Rossmann P, Hrnčíř T, Kverka M, Zákostelská Z, Klimešová K, Přibylová J, Bártová J, Sanchez D, Fundová P, Borovská D, Srůtková D, Zídek Z, Schwarzer M, Drastich P, Funda DP. Tlaskalová-Hogenová H, et al. Cell Mol Immunol. 2011 Mar;8(2):110-20. doi: 10.1038/cmi.2010.67. Epub 2011 Jan 31. Cell Mol Immunol. 2011. PMID: 21278760 Free PMC article. Review.
Cited by
- Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer.
Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, Park KK, Hu Y, Chung WY, Song NY. Park SY, et al. Cancers (Basel). 2021 Apr 28;13(9):2124. doi: 10.3390/cancers13092124. Cancers (Basel). 2021. PMID: 33924899 Free PMC article. Review. - Intestinal microbiota succession and immunomodulatory consequences after introduction of Lactobacillus reuteri I5007 in neonatal piglets.
Hou C, Liu H, Zhang J, Zhang S, Yang F, Zeng X, Thacker PA, Zhang G, Qiao S. Hou C, et al. PLoS One. 2015 Mar 16;10(3):e0119505. doi: 10.1371/journal.pone.0119505. eCollection 2015. PLoS One. 2015. PMID: 25775260 Free PMC article. - Impact of maternal nutrition in pregnancy and lactation on offspring gut microbial composition and function.
Chu DM, Meyer KM, Prince AL, Aagaard KM. Chu DM, et al. Gut Microbes. 2016 Nov;7(6):459-470. doi: 10.1080/19490976.2016.1241357. Epub 2016 Sep 29. Gut Microbes. 2016. PMID: 27686144 Free PMC article. Review. - Antibiotic resistance and host immune system-induced metal bactericidal control are key factors for microbial persistence in the developing human preterm infant gut microbiome.
Peters SL, Morowitz MJ, Hettich RL. Peters SL, et al. Front Microbiol. 2022 Nov 21;13:958638. doi: 10.3389/fmicb.2022.958638. eCollection 2022. Front Microbiol. 2022. PMID: 36478853 Free PMC article. - Infant gut microbiome composition correlated with type 1 diabetes acquisition in the general population: the ABIS study.
Bélteky M, Milletich PL, Ahrens AP, Triplett EW, Ludvigsson J. Bélteky M, et al. Diabetologia. 2023 Jun;66(6):1116-1128. doi: 10.1007/s00125-023-05895-7. Epub 2023 Mar 25. Diabetologia. 2023. PMID: 36964264
References
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr. 1999;69:1046S–1051S. - PubMed
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunology Letters. 2004;93:97–108. - PubMed
- Pozzilli P, Signore A, Williams AJK, Beales PE. Nod Mouse Colonies Around the World - Recent Facts and Figures. Immunology Today. 1993;14:193–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources