Mitochondrial Ca(2+) and apoptosis - PubMed (original) (raw)
Review
doi: 10.1016/j.ceca.2012.02.008. Epub 2012 Apr 3.
Federica Baldassari, Angela Bononi, Massimo Bonora, Elena De Marchi, Saverio Marchi, Sonia Missiroli, Simone Patergnani, Alessandro Rimessi, Jan M Suski, Mariusz R Wieckowski, Paolo Pinton
Affiliations
- PMID: 22480931
- PMCID: PMC3396846
- DOI: 10.1016/j.ceca.2012.02.008
Review
Mitochondrial Ca(2+) and apoptosis
Carlotta Giorgi et al. Cell Calcium. 2012 Jul.
Abstract
Mitochondria are key decoding stations of the apoptotic process. In support of this view, a large body of experimental evidence has unambiguously revealed that, in addition to the well-established function of producing most of the cellular ATP, mitochondria play a fundamental role in triggering apoptotic cell death. Various apoptotic stimuli cause the release of specific mitochondrial pro-apoptotic factors into the cytosol. The molecular mechanism of this release is still controversial, but there is no doubt that mitochondrial calcium (Ca(2+)) overload is one of the pro-apoptotic ways to induce the swelling of mitochondria, with perturbation or rupture of the outer membrane, and in turn the release of mitochondrial apoptotic factors into the cytosol. Here, we review as different proteins that participate in mitochondrial Ca(2+) homeostasis and in turn modulate the effectiveness of Ca(2+)-dependent apoptotic stimuli. Strikingly, the final outcome at the cellular level is similar, albeit through completely different molecular mechanisms: a reduced mitochondrial Ca(2+) overload upon pro-apoptotic stimuli that dramatically blunts the apoptotic response.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
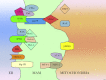
Fig. 1
Schematic intracellular distribution of the reported proteins involved in ER–mitochondria Ca2+ cross-talk. Abbreviations: AKT, v-akt murine thymoma viral oncogene homologue; BAX, Bcl-2-associated X; Bcl-xL, B-cell lymphomas extra long; Bcl-2, B-cell lymphomas 2; BIK, Bcl-2 interacting killer; ER, endoplasmic reticulum; FHIT, fragile histidine triad; grp75, glucose-regulated protein 75; IP3R, inositol 1,4,5-trisphosphate receptor; MAMs, mitochondria-associated membranes; mcl-1, myeloid cell leukaemia-1; MFN-1/-2, mitofusin-1, -2; p66Shc, 66-kDa isoform of the growth factor adapter shc; PML, promyelocytic leukaemia protein; PP2a, protein phosphatase 2a; SERCA, sarco-endoplasmic reticulum Ca2+ ATPase; Sig-1R, Sigma-1 receptor; STAT, Signal Transducer and Activator of Transcription; VDAC, voltage-dependent anion channel.
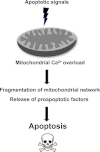
Fig. 2
Schematic representation of mitochondrial Ca2+-dependent apoptosis.
Similar articles
- Enhanced inter-compartmental Ca2+ flux modulates mitochondrial metabolism and apoptotic threshold during aging.
Madreiter-Sokolowski CT, Waldeck-Weiermair M, Bourguignon MP, Villeneuve N, Gottschalk B, Klec C, Stryeck S, Radulovic S, Parichatikanond W, Frank S, Madl T, Malli R, Graier WF. Madreiter-Sokolowski CT, et al. Redox Biol. 2019 Jan;20:458-466. doi: 10.1016/j.redox.2018.11.003. Epub 2018 Nov 9. Redox Biol. 2019. PMID: 30458321 Free PMC article. - Porcine Circovirus Type 2 Induces ORF3-Independent Mitochondrial Apoptosis via PERK Activation and Elevation of Cytosolic Calcium.
Zhang Y, Sun R, Geng S, Shan Y, Li X, Fang W. Zhang Y, et al. J Virol. 2019 Mar 21;93(7):e01784-18. doi: 10.1128/JVI.01784-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651358 Free PMC article. - Bcl-2-family proteins and the role of mitochondria in apoptosis.
Kuwana T, Newmeyer DD. Kuwana T, et al. Curr Opin Cell Biol. 2003 Dec;15(6):691-9. doi: 10.1016/j.ceb.2003.10.004. Curr Opin Cell Biol. 2003. PMID: 14644193 Review. - Alzheimer's disease via enhanced calcium signaling caused by the decrease of endoplasmic reticulum-mitochondrial distance.
Qi H, Shuai J. Qi H, et al. Med Hypotheses. 2016 Apr;89:28-31. doi: 10.1016/j.mehy.2016.01.022. Epub 2016 Feb 6. Med Hypotheses. 2016. PMID: 26968904 - Mitochondrial VDAC, the Na+/Ca2+ Exchanger, and the Ca2+ Uniporter in Ca2+ Dynamics and Signaling.
Shoshan-Barmatz V, De S. Shoshan-Barmatz V, et al. Adv Exp Med Biol. 2017;981:323-347. doi: 10.1007/978-3-319-55858-5_13. Adv Exp Med Biol. 2017. PMID: 29594867 Review.
Cited by
- The antimicrobial peptide LL-37 alters human osteoblast Ca2+ handling and induces Ca2+-independent apoptosis.
Säll J, Carlsson M, Gidlöf O, Holm A, Humlén J, Ohman J, Svensson D, Nilsson BO, Jönsson D. Säll J, et al. J Innate Immun. 2013;5(3):290-300. doi: 10.1159/000346587. Epub 2013 Feb 13. J Innate Immun. 2013. PMID: 23406612 Free PMC article. - Endoplasmic Reticulum-Mitochondria Crosstalk and Beta-Cell Destruction in Type 1 Diabetes.
Vig S, Lambooij JM, Zaldumbide A, Guigas B. Vig S, et al. Front Immunol. 2021 Apr 16;12:669492. doi: 10.3389/fimmu.2021.669492. eCollection 2021. Front Immunol. 2021. PMID: 33936111 Free PMC article. Review. - Recent Advances and Therapeutic Implications of 2-Oxoglutarate-Dependent Dioxygenases in Ischemic Stroke.
Xie J, Zhang Z. Xie J, et al. Mol Neurobiol. 2024 Jul;61(7):3949-3975. doi: 10.1007/s12035-023-03790-1. Epub 2023 Dec 2. Mol Neurobiol. 2024. PMID: 38041714 Review. - NS5A Promotes Constitutive Degradation of IP3R3 to Counteract Apoptosis Induced by Hepatitis C Virus.
Kuchay S, Saeed M, Giorgi C, Li J, Hoffmann HH, Pinton P, Rice CM, Pagano M. Kuchay S, et al. Cell Rep. 2018 Oct 23;25(4):833-840.e3. doi: 10.1016/j.celrep.2018.09.088. Cell Rep. 2018. PMID: 30355490 Free PMC article. - Secondary Mechanisms of Neurotrauma: A Closer Look at the Evidence.
Aghili-Mehrizi S, Williams E, Yan S, Willman M, Willman J, Lucke-Wold B. Aghili-Mehrizi S, et al. Diseases. 2022 May 23;10(2):30. doi: 10.3390/diseases10020030. Diseases. 2022. PMID: 35645251 Free PMC article. Review.
References
- Alnemri E.S., Livingston D.J., Nicholson D.W., Salvesen G., Thornberry N.A., Wong W.W., Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
- Nicholson D.W., Thornberry N.A. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. - PubMed
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. - PubMed
- Thornberry N.A., Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous