Sex differences in molecular and cellular substrates of stress - PubMed (original) (raw)
Review
Sex differences in molecular and cellular substrates of stress
Debra A Bangasser et al. Cell Mol Neurobiol. 2012 Jul.
Abstract
Women are twice as likely as men to suffer from stress-related psychiatric disorders, like unipolar depression and post-traumatic stress disorder. Although the underlying neural mechanisms are not well characterized, the pivotal role of stress in the onset and severity of these diseases has led to the idea that sex differences in stress responses account for this sex bias. Corticotropin-releasing factor (CRF) orchestrates stress responses by acting both as a neurohormone to initiate the hypothalamic-pituitary-adrenal (HPA) axis and as a neuromodulator in the brain. One target of CRF modulation is the locus coeruleus (LC)-norepinephrine system, which coordinates arousal components of the stress response. Hypersecretion of CRF and dysregulation of targets downstream from CRF, such as the HPA axis and LC-norepinephrine system, are characteristic features of many stress-related psychiatric diseases, suggesting a causal role for CRF and its targets in the development of these disorders. This review will describe sex differences in CRF and the LC-norepinephrine system that can increase stress sensitivity in females, making them vulnerable to stress-related disorders. Evidence for gonadal hormone regulation of hypothalamic CRF is discussed as an effect that can lead to increased HPA axis activity in females. Sex differences in the structure of LC neurons that create the potential for hyperarousal in response to emotional stimuli are described. Finally, sex differences at the molecular level of the CRF(1) receptor that make the LC-norepinephrine system more reactive in females are reviewed. The implications of these sex differences for the treatment of stress-related psychiatric disorders also will be discussed.
Figures
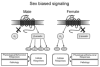
Figure 1
This schematic depicts the predicted model of sex biased signaling. In males the CRF1 receptor associates with β-arrestin2, which sterically hinders Gs association and biases signaling towards β-arrestin2-related pathways such as NFκB, AKT, ERK, and Src. In females, the CRF1 receptor does not associate with β-arrestin2 as well and is more highly coupled to Gs, which signals through cAMP and sometimes ERK. Note that in both males and females CRF activates the receptor, but the signaling events are unique because of sex specific interactions of CRF1 receptors with binding partners. The differential cellular responses can translate to different physiological and behavioral responses to the same stressor and different pathology.
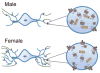
Figure 2
This schematic illustrates sex differences in the LC-arousal system. The images on the left depict LC neurons with dendrites (blue) receiving afferent inputs (in green). Because the LC dendritic tree is longer and more complex in females, they are more likely to receive projections from afferent structures that terminate in the peri-LC. The images on the right depict magnified views of LC dendrites in order to illustrate sex differences in the CRF1 receptor coupling and trafficking. In males, CRF1 receptors (red) associate with β-arrestin2 following ligand binding and become localized in the cytoplasm. CRF1 receptors of females are highly coupled to Gs and following ligand binding they are predominantly found on the plasma membrane.
Similar articles
- Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression.
Bangasser DA, Reyes BA, Piel D, Garachh V, Zhang XY, Plona ZM, Van Bockstaele EJ, Beck SG, Valentino RJ. Bangasser DA, et al. Mol Psychiatry. 2013 Feb;18(2):166-73. doi: 10.1038/mp.2012.24. Epub 2012 Apr 17. Mol Psychiatry. 2013. PMID: 22508464 Free PMC article. - Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology.
Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. Bangasser DA, et al. Mol Psychiatry. 2010 Sep;15(9):877, 896-904. doi: 10.1038/mp.2010.66. Epub 2010 Jun 15. Mol Psychiatry. 2010. PMID: 20548297 Free PMC article. - Sex differences in stress responses: a critical role for corticotropin-releasing factor.
Bangasser DA, Wiersielis KR. Bangasser DA, et al. Hormones (Athens). 2018 Mar;17(1):5-13. doi: 10.1007/s42000-018-0002-z. Epub 2018 Apr 16. Hormones (Athens). 2018. PMID: 29858858 Review. - Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress.
Bangasser DA, Wiersielis KR, Khantsis S. Bangasser DA, et al. Brain Res. 2016 Jun 15;1641(Pt B):177-88. doi: 10.1016/j.brainres.2015.11.021. Epub 2015 Nov 21. Brain Res. 2016. PMID: 26607253 Free PMC article. Review. - Molecular and cellular sex differences at the intersection of stress and arousal.
Valentino RJ, Reyes B, Van Bockstaele E, Bangasser D. Valentino RJ, et al. Neuropharmacology. 2012 Jan;62(1):13-20. doi: 10.1016/j.neuropharm.2011.06.004. Epub 2011 Jun 21. Neuropharmacology. 2012. PMID: 21712048 Free PMC article. Review.
Cited by
- Adolescent social isolation increases cocaine seeking in male and female mice.
Fosnocht AQ, Lucerne KE, Ellis AS, Olimpo NA, Briand LA. Fosnocht AQ, et al. Behav Brain Res. 2019 Feb 1;359:589-596. doi: 10.1016/j.bbr.2018.10.007. Epub 2018 Oct 5. Behav Brain Res. 2019. PMID: 30296530 Free PMC article. - Effects of Psychological and Lifestyle Factors on Metabolic Syndrome Following the Fukushima Daiichi Nuclear Power Plant Accident: The Fukushima Health Management Survey.
Takahashi A, Ohira T, Okazaki K, Yasumura S, Sakai A, Maeda M, Yabe H, Hosoya M, Ohtsuru A, Kawasaki Y, Shimabukuro M, Kazama J, Hashimoto S, Watanabe K, Nakano H, Hayashi F, Ohto H, Kamiya K, Ohira H. Takahashi A, et al. J Atheroscler Thromb. 2020 Sep 1;27(9):1010-1018. doi: 10.5551/jat.52225. Epub 2020 Jan 31. J Atheroscler Thromb. 2020. PMID: 32009075 Free PMC article. - Gender differences in African Americans' reactions to and coping with discrimination: Results from The National Study of American Life.
Sullivan JM, Harman M, Sullivan S. Sullivan JM, et al. J Community Psychol. 2021 Sep;49(7):2424-2440. doi: 10.1002/jcop.22677. Epub 2021 Jul 28. J Community Psychol. 2021. PMID: 34320229 Free PMC article. - A putative causal relationship between genetically determined female body shape and posttraumatic stress disorder.
Polimanti R, Amstadter AB, Stein MB, Almli LM, Baker DG, Bierut LJ, Bradley B, Farrer LA, Johnson EO, King A, Kranzler HR, Maihofer AX, Rice JP, Roberts AL, Saccone NL, Zhao H, Liberzon I, Ressler KJ, Nievergelt CM, Koenen KC, Gelernter J; Psychiatric Genomics Consortium Posttraumatic Stress Disorder Workgroup. Polimanti R, et al. Genome Med. 2017 Nov 27;9(1):99. doi: 10.1186/s13073-017-0491-4. Genome Med. 2017. PMID: 29178946 Free PMC article. - Corticotropin releasing hormone receptor 1 in the medial prefrontal cortex mediates aversion resistant alcohol intake.
Arnold ME, Harber CE, Beugelsdyk LA, Decker Ramirez EB, Phillips GB, Schank JR. Arnold ME, et al. Psychopharmacology (Berl). 2024 Dec;241(12):2539-2550. doi: 10.1007/s00213-024-06707-5. Epub 2024 Oct 28. Psychopharmacology (Berl). 2024. PMID: 39466414
References
- Abercrombie ED, Keller RW, Zigmond MJ. Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience. 1988;27:897–904. - PubMed
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, Moorjani P, Haddad S, Basu A, Fagerness J, Saxe G, Smoller JW, Koenen KC. Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers. 2011;30 (2–3):89–99. doi: 10.3233/DMA-2011-0761. D7566715733N1LM2 [pii] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R00 MH092438/MH/NIMH NIH HHS/United States
- MH092438/MH/NIMH NIH HHS/United States
- K99 MH092438/MH/NIMH NIH HHS/United States
- MH40008/MH/NIMH NIH HHS/United States
- R01 MH040008/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical