Disabled-2 (Dab2) inhibits Wnt/β-catenin signalling by binding LRP6 and promoting its internalization through clathrin - PubMed (original) (raw)
Disabled-2 (Dab2) inhibits Wnt/β-catenin signalling by binding LRP6 and promoting its internalization through clathrin
Yong Jiang et al. EMBO J. 2012.
Abstract
Canonical Wnt signalling requires caveolin-dependent internalization of low-density lipoprotein receptor-related protein 6 (LRP6). Here we report that the tumour suppressor and endocytic adaptor disabled-2 (Dab2), previously described as an inhibitor of Wnt/β-catenin signalling, selectively recruits LRP6 to the clathrin-dependent endocytic route, thereby sequestering it from caveolin-mediated endocytosis. Wnt stimulation induces the casein kinase 2 (CK2)-dependent phosphorylation of LRP6 at S1579, promoting its binding to Dab2 and internalization with clathrin. LRP6 receptor mutant (S1579A), deficient in CK2-mediated phosphorylation and Dab2 binding, fails to associate with clathrin, and thus escapes the inhibitory effects of Dab2 on Wnt/β-catenin signalling. Our data suggest that the S1579 site of LRP6 is a negative regulatory point during LRP6-mediated dorsoventral patterning in zebrafish and in allograft mouse tumour models. We conclude that the tumour suppressor functions of Dab2 involve modulation of canonical Wnt signalling by regulating the endocytic fate of the LRP6 receptor.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Figure 1
Caveolin-mediated endocytosis is essential for Wnt/β-catenin signalling and Dab2 has no effect on the internalization rate of LRP6. (A) Increasing concentrations of DN K44A were co-transfected into F9 cells with 0.5 μg of TOP/FOPFlash luciferase reporter construct and luciferase activity assays were performed and quantitated. (B) F9 and F9-Dab2 cells were transfected with 0.5 μg of TOP/FOPFlash luciferase reporter constructs. Cells were pre-treated for 1 h with the indicated concentration of nystatin or MDC prior to Wnt3A stimulation and luciferase activity determination. (C) F9 and F9-Dab2 cells were treated with Wnt3A conditioned media for the indicated times, and whole-cell lysates (WCL) were immunoblotted with α–β-catenin, α-cyclin D1 and α-Dab2 antibodies. α-Hsp90 immunoblot is used as a loading control. (D) F9 and (E) F9-Dab2 cells were pre-treated with nystatin (100 μg/ml) or MDC (50 μM) for 1 h prior to Wnt3A stimulation for the indicated times. WCL were immunoblotted with α–β-catenin and α-cyclin D1 antibodies. (F) F9 and F9-Dab2 cell surface proteins were biotinylated using a reversible biotinylation agent (cleavable Sulfo-NHS-SS-Biotin). Following labelling, cells were stimulated with Wnt3A for the indicated times at 37°C and cells were split into two groups. WCL from one group (non-reduced) were precipitated with avidin-agarose beads followed by immunoblot analysis with α-LRP6 antibody. The other group of cells (reduced) was treated with a glutathione-containing solution to strip away any biotinylated proteins remaining on the cell surface. WCL were prepared and internalized cell surface proteins protected from biotin stripping were precipitated with avidin-agarose beads followed by immunoblotting with α-LRP6 antibody. EGF receptor endocytosis was used as control (immunoblotting with α-EGF receptor antibody). (G) F9 and F9-Dab2 cells were stimulated with Wnt3A for the times indicated and placed at 4°C for a 1 h labelling with a biotinylation agent (non-cleavable Sulfo-NHS-LC-Biotin). WCL were prepared and biotin-labelled cell surface proteins were precipitated with avidin-agarose beads and analysed by immunoblotting with α-LRP6 antibody. Figure source data can be found with the Supplementary data.
Figure 2
Dab2 shunts LRP6 towards clathrin-dependent endocytosis. (A) Flag-tagged LRP6 (10 μg) was transfected into F9 and F9-Dab2 cells. Following transfection, cells were treated with Wnt3A for the times indicated and WCL were immunoprecipitated with control α-IgG or α-Flag antibody. Immunocomplexes were subjected to immunoblot analysis with α-caveolin, α-clathrin and α-Flag antibodies. WCL were also immunoblotted with α-Dab2 antibody to demonstrate relative Dab2 levels. (B) Flag-tagged LRP6 (10 μg) was transfected into F9 Dab2 cells and, following transfection, stimulated with Wnt3A for the indicated times. WCL were subjected to immunoprecipitation with α-clathrin, α-caveolin and α-Flag antibodies. Immunocomplexes were immunoblotted with α-Dab2 antibody to detect clathrin/Dab2, caveolin/Dab2 and Flag-LRP6/Dab2 interactions. WCL were also immunoblotted with α-Dab2 antibody for demonstrating relative Dab2 levels. IgG antisera was used as negative control for the various immunoprecipitations. (C) WCL from F9 and F9-Dab2 cells treated with or without Wnt3A for 1 h were fractionated by sucrose density gradient centrifugation. Fractions were collected and aliquots of each were analysed by immunoblotting with α-caveolin, α-clathrin, α-LRP6 and α-p-LRP6 (S1490). (D) Pooled sucrose gradient fractions (light fractions no. 3–5 and heavy fractions no. 8–10) were immunoprecipitated with α-caveolin (left panels) and α-clathrin (right panels) antibodies. Immunocomplexes were immunoblotted with α-LRP6, α-Axin, α-GSK3β, α-Dab2, and α-caveolin or α-clathrin. IgG antisera was used as negative control for the various immunoprecipitations. Figure source data can be found with the Supplementary data.
Figure 3
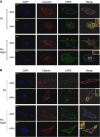
Subcellular distribution and co-localization of LRP6/caveolin and LRP6/clathrin in the absence and presence of Dab2. Immunofluorescence of caveolin and LRP6 (A) and clathrin and LRP6 (B) in F9 and F9-Dab2 cells treated with or without Wnt3A for 1 h. After treatment all cells were fixed and stained with α-LRP6, α-caveolin or α-clathrin antibodies. LRP6 is shown in green, and caveolin and clathrin are shown in red. Co-localization of LRP6 with caveolin or clathrin is depicted by the yellow in the panels labelled ‘merge’. DAPI was used to stain the nuclei. Scale bars, 5 μm.
Figure 4
Dab2 interacts with LRP6. (A) Schematic of the VSVG-tagged LRP6 intracellular constructs utilized. The five reiterated PPPS/TP motifs are designated as a, b, c, d and e, and the asterisks (*) indicate an S or T to A substitution. ‘TM’ indicates the position of the transmembrane region of LRP6. Lower panels: all constructs were transfected into F9-Dab2 cells and WCL were prepared and subjected to immunoprecipitation with either α-VSVG antibody or α-IgG antisera (as negative control). Immunocomplexes were analysed by immunoblotting with α-Dab2 antibody to detect LRP6/Dab2 interactions and with α-VSVG to detect the expression of the various constructs. (B) Schematic of various Flag-tagged Dab2 constructs utilized. Lower panels: Dab2 constructs were transfected into F9 cells and, following transfection, treated with Wnt3A for 2 h. WCL were subjected to immunoprecipitation analysis with α-Flag antibody and α-IgG antisera (as negative control). Immunocomplexes were analysed by immunoblotting with α-LRP6 to detect Dab2/LRP6 interactions and with α-Flag to detect the expression of the various Dab2 constructs. (C) VSVG-tagged LRP6 intracellular mutant constructs ΔN, ΔN1 and ΔNG were transfected into F9 cells and treated with Wnt3A for the times indicated. WCL were subjected to GST-PTB pulldown assays (GST fusion protein with the N-terminal PTB domain of Dab2). The precipitated complexes were analysed by immunoblotting with α-VSVG to detect PTB/LRP6 interactions. (D) Schematic of the intracellular domain of LRP6 and of the various phospho-mutant sites generated. These phospho-mutants were generated in the context of full-length LRP6 and cloned in Flag-pCS2 expression vector. Also depicted is the consensus CK2 phosphorylation site ‘SAEE’ in LRP6. Constructs were transfected into F9 Dab2 cells and following transfection treated with Wnt3A for 2 h. WCL were immnunoprecipitated with α-Flag antibody and α-IgG antisera (as negative control). Immunocomplexes were analysed by immunoblotting with α-Dab2 to detect Dab2/LRP6 interactions and with α-Flag to detect the expression of the various Dab2 constructs. (E) Flag-tagged WT and phospho-mutant S1579A LRP6 constructs were transfected into F9 Dab2 cells. Following transfection, cells were pre-treated with apigenin (10 and 20 μM) for 30 min prior to a 2 h Wnt3A stimulation. WCL were immunoprecipitated with α-Flag antibody followed by immunoblotting with α-Dab2, α-clathrin and α-caveolin. (F) siDab2 F9 cells, WT F9 cells, control shRNA F9 cells and CK2 shRNA F9 cells were treated with 100 nM RA for 3 days. Following treatment, cells were stimulated±Wnt3A for 2 h and WCL were immunoprecipitated with α-clathrin. The precipitated complexes were analysed by immunoblotting with α-Dab2 and α-LRP6 to detect clathrin/LRP6 and clathrin/Dab2 interactions. (G) Relative protein expression in the cells in (F) was detected by immunoblotting with α-LRP6, α-Dab2, α-clathrin and α-CK2 antibodies. Figure source data can be found with the Supplementary data.
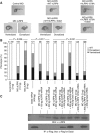
Figure 5
Dab2 fails to attenuate phospho-mutant S1579A LRP6-mediated dorsoventral patterning during zebrafish embryonic development. (A) Left panels: Control (MO) and zebrafish LRP6 anti-sense (zLRP6) morpholinos (3 ng) were injected into one-cell-stage fertilized zebrafish embryos and 24 h post fertilization (hpf) photos were taken using a Leica MZ95 dissection microscope. Injection of zLRP6 MO yielded the dorsoventralized phenotypes. Middle panels: Injection of zLRP6 MO (3 ng) together with human LRP6 mRNA (hLRP6; 300 pg) into one-cell-stage zebrafish embryos rescued the dorsoventralized phenotypes (MO zLRP6+WT hLRP6). Injection of zLRP6 MO (3 ng) and hLRP6 mRNA (300 pg) and Dab2 mRNA (400 pg) resulted in the dorsoventral phenotypes. Right panels: Co-injection of zLRP6 MO (3 ng) with the hLRP6 phospho-mutant S1579A (hLRP6 1579A; 300 pg) rescued the dorsoventralized phenotypes, and injection of these together with Dab2 (zLRP6 MO+hLRP6 1579A+Dab2) failed to cause the dorsoventralized phenotypes. (B) Statistical analysis of the results from the injection assay of panel A. (C) A set of injected embryos described in (A) were collected and proteins were extracted, followed by immunoblot and immunoprecipitation analysis to detect expression levels of LRP6 and Dab2 using α-LRP6 and α-Flag antibodies. Figure source data can be found with the Supplementary data.
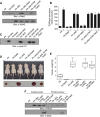
Figure 6
Dab2 fails to attenuate phospho-mutant S1579A LRP6-mediated tumourigenesis. (A) WCL from the indicated stably transfected cell lines were analysed by immunoblot analysis using α-Dab2 and α-VSVG antibodies to detect the expression levels of Dab2 and the intracellular domain of LRP6, respectively. (B) The relative TOP/FOPFlash luciferase activities and (C) expression levels of cyclin D1 in the stably transfected cell lines stimulated with or without Wnt3A were determined. Data are presented as means±s.d. for three independent experiments (_n_=3), each experiment being performed in triplicate. Cyclin D1 expression levels were determined by immunoblot analysis using α-cyclin D1 antibody. (D) Subcutaneous inoculation of 5×105 cells of the indicated stably transfected cell lines in the left hind flank of 6-week-old BalbC athymic nude mice (nu/nu) was performed. Each animal was also inoculated with 5×105 cells of the F9-Dab2 cell line in the right hind flank serving as a control. Images were taken 45 days post injection. (E) Tumours were excised and tumour weight was evaluated as a box-and-whisker plot to analyse differences between mean tumour weights among the various cells used. Data are presented as means±s.e.m. for _n_=10 samples per group. (F) Protein extracts from cultured cells established from the excised tumours (cultured cells) or the primary tumours themselves (primary tumours) were analysed by immunoblot analysis using α-VSVG and α-Dab2 to detect relative expression levels of Dab2 and VSVG-tagged ΔNG and ΔNG S1579A. Figure source data can be found with the Supplementary data.
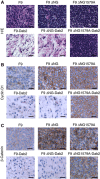
Figure 7
IHC analysis of tumour tissues. (A) H&E staining of excised tumours from mice inoculated with the indicated cell lines. IHC analysis of paraffin sections from the excised tumours was performed with α-cyclin D1 (B) and α–β-catenin (C). Scale bars, 50 μm.
Figure 8
Knockdown of CK2 inhibits the tumour suppressor functions of Dab2. (A) Left panels: F9-Dab2 stably expressing control shRNA or CK2 shRNA were generated as described in Materials and methods and analysed by immunoblot analysis for expression levels of CK2, LRP6 and Dab2. Middle panels: Dab2 immunoprecipitates from F9-Dab2 stably expressing control shRNA or CK2 shRNA treated±Wnt3A were also analysed for co-immunoprecipitation of LRP6. Right panel: Wnt3A-stimulated TOP/FOPFlash luciferase activity was determined in parental F9 cells, F9-Dab2-expressing cells with control shRNA and F9-Dab2-expressing cells in which CK2 is stably attenuated (F9-Dab2 CK2 KD). (B) Subcutaneous inoculation of F9-Dab2 shCK2 cells (5×105) in the right hind flank of 6-week-old BalbC athymic nude mice (nu/nu) was performed. Each animal was also inoculated with 5×105 F9-Dab2 cells with control shRNA in the left hind flank to serve as a control. Images were taken 45 days post injection. Tumours were excised (middle panel) and tumour weight was evaluated as a box-and-whisker plot (right panel) to analyse the differences between mean tumour weights. Data are presented as means±s.e.m. for _n_=10 samples per group. (C) Model depicting the shunting of LRP6 towards the clathrin endocytic pathway by Dab2. In the absence of Dab2, LRP6 is internalized through the caveolin pathway, resulting in β-catenin accumulation, whereas in its presence LRP6 is internalized through the clathrin pathway and fails to inhibit the β-catenin destruction complex. Figure source data can be found with the Supplementary data.
Similar articles
- Dab2 stabilizes Axin and attenuates Wnt/beta-catenin signaling by preventing protein phosphatase 1 (PP1)-Axin interactions.
Jiang Y, Luo W, Howe PH. Jiang Y, et al. Oncogene. 2009 Aug 20;28(33):2999-3007. doi: 10.1038/onc.2009.157. Epub 2009 Jul 6. Oncogene. 2009. PMID: 19581931 Free PMC article. - Clathrin and AP2 are required for PtdIns(4,5)P2-mediated formation of LRP6 signalosomes.
Kim I, Pan W, Jones SA, Zhang Y, Zhuang X, Wu D. Kim I, et al. J Cell Biol. 2013 Feb 18;200(4):419-28. doi: 10.1083/jcb.201206096. Epub 2013 Feb 11. J Cell Biol. 2013. PMID: 23400998 Free PMC article. - Down-regulation of Wt1 activates Wnt/β-catenin signaling through modulating endocytic route of LRP6 in podocyte dysfunction in vitro.
Jing Z, Wei-jie Y, Yi-Feng ZG. Jing Z, et al. Cell Signal. 2015 Sep;27(9):1772-80. doi: 10.1016/j.cellsig.2015.05.018. Epub 2015 Jun 3. Cell Signal. 2015. PMID: 26049137 - β-Catenin-Independent Roles of Wnt/LRP6 Signaling.
Acebron SP, Niehrs C. Acebron SP, et al. Trends Cell Biol. 2016 Dec;26(12):956-967. doi: 10.1016/j.tcb.2016.07.009. Epub 2016 Aug 24. Trends Cell Biol. 2016. PMID: 27568239 Review. - LRP6 Receptor Plays Essential Functions in Development and Human Diseases.
Alrefaei AF, Abu-Elmagd M. Alrefaei AF, et al. Genes (Basel). 2022 Jan 10;13(1):120. doi: 10.3390/genes13010120. Genes (Basel). 2022. PMID: 35052459 Free PMC article. Review.
Cited by
- Tyrosine-based signal mediates LRP6 receptor endocytosis and desensitization of Wnt/β-catenin pathway signaling.
Liu CC, Kanekiyo T, Roth B, Bu G. Liu CC, et al. J Biol Chem. 2014 Oct 3;289(40):27562-70. doi: 10.1074/jbc.M113.533927. Epub 2014 Aug 20. J Biol Chem. 2014. PMID: 25143377 Free PMC article. - Integrin-beta3 clusters recruit clathrin-mediated endocytic machinery in the absence of traction force.
Yu CH, Rafiq NB, Cao F, Zhou Y, Krishnasamy A, Biswas KH, Ravasio A, Chen Z, Wang YH, Kawauchi K, Jones GE, Sheetz MP. Yu CH, et al. Nat Commun. 2015 Oct 28;6:8672. doi: 10.1038/ncomms9672. Nat Commun. 2015. PMID: 26507506 Free PMC article. - Cholesterol activates the Wnt/PCP-YAP signaling in SOAT1-targeted treatment of colon cancer.
Xu H, Xia H, Zhou S, Tang Q, Bi F. Xu H, et al. Cell Death Discov. 2021 Feb 26;7(1):38. doi: 10.1038/s41420-021-00421-3. Cell Death Discov. 2021. PMID: 33637695 Free PMC article. - Multi-omics characterization of the monkeypox virus infection.
Huang Y, Bergant V, Grass V, Emslander Q, Hamad MS, Hubel P, Mergner J, Piras A, Krey K, Henrici A, Öllinger R, Tesfamariam YM, Dalla Rosa I, Bunse T, Sutter G, Ebert G, Schmidt FI, Way M, Rad R, Bowie AG, Protzer U, Pichlmair A. Huang Y, et al. Nat Commun. 2024 Aug 8;15(1):6778. doi: 10.1038/s41467-024-51074-6. Nat Commun. 2024. PMID: 39117661 Free PMC article. - High-affinity Dkk1 receptor Kremen1 is internalized by clathrin-mediated endocytosis.
Mishra SK, Funair L, Cressley A, Gittes GK, Burns RC. Mishra SK, et al. PLoS One. 2012;7(12):e52190. doi: 10.1371/journal.pone.0052190. Epub 2012 Dec 14. PLoS One. 2012. PMID: 23251700 Free PMC article.
References
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C (2007) Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622 - PubMed
- Bonifacino JS, Traub LM (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447 - PubMed
- Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–480 - PubMed
- Davidson G, Wu W, Shen J, Bilic J, Fenger U, Stannek P, Glinka A, Niehrs C (2005) Casein kinase 1 gamma couples Wnt receptor activation to cytoplasmic signal transduction. Nature 438: 867–872 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases