Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns - PubMed (original) (raw)
Comparative Study
Coincident generation of pyramidal neurons and protoplasmic astrocytes in neocortical columns
Sanjay Magavi et al. J Neurosci. 2012.
Abstract
Astrocytes, one of the most common cell types in the brain, are essential for processes ranging from neural development through potassium homeostasis to synaptic plasticity. Surprisingly, the developmental origins of astrocytes in the neocortex are still controversial. To investigate the patterns of astrocyte development in the neocortex we examined cortical development in a transgenic mouse in which a random, sparse subset of neural progenitors undergoes CRE/lox recombination, permanently labeling their progeny. We demonstrate that neural progenitors in neocortex generate discrete columnar structures that contain both projection neurons and protoplasmic astrocytes. Ninety-five percent of developmental cortical columns labeled in our system contained both astrocytes and neurons. The astrocyte to neuron ratio of labeled cells in a developmental column was 1:7.4, similar to the overall ratio of 1:8.4 across the entire gray matter of the neocortex, indicating that column-associated astrocytes account for the majority of protoplasmic astrocytes in neocortex. Most of the labeled columns contained multiple clusters of several astrocytes. Dividing cells were found at the base of neuronal columns at the beginning of gliogenesis, and later within the cortical layers, suggesting a mechanism by which astrocytes could be distributed within a column. These data indicate that radial glia are the source of both neurons and astrocytes in the neocortex, and that these two cell types are generated in a spatially restricted manner during cortical development.
Figures
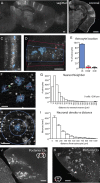
Figure 1.
Developmentally generated columns of cells in cortex contain both neurons and astrocytes. A, B, Adult TFC.09 × Z/EG mice exhibit radially arrayed columns of cells in cortex apparent both along the sagittal (A) and coronal (B) planes. These columns of cells display the characteristic morphologies of developmental columns generated in previous cortical clonal labeling experiments using other methods. C, These columns of GFP labeled cells (white) contained both neurons and astrocytes, which have been pseudocolored blue for the purposes of visualization. C, D, F, G, We measured whether GFP+ astrocytes were in (blue lines), near (within 50 μm as indicated by the red lines), or outside of GFP+ columns. Columns were defined as discreet, elongated structures in the cortex that contained GFP+ cells with unambiguous pyramidal neuron morphologies spanning from the ventricular to the pial surface. F, G, We measured the distance between astrocytes (blue) and their nearest neighboring neuron (green) to assess their spatial relationship. The average distance between an astrocyte and its nearest neighbor was 33 ± 28 μm. H, I, We assessed neuronal density as a function of distance, measuring the density of neurons within 100 μm of an astrocyte, 200 μm, 300 μm, etc. Astrocytes were found in regions of high neuronal density, as would be expected of astrocytes located within neuronal columns. J, Astrocytes were found associated with neuronal columns even when the columns were curved, as they are commonly in the occipital cortex, suggesting their association is not merely accidental. K, In piriform cortex, with its three-layered structure that is distinct from neocortex, neither layer 2 interneurons nor astrocytes exhibited columnar organization, demonstrating that the columnar structures we found in neocortex are unlikely to be artifactual. Scale bars: A, B, M, N, 500 μm; D, J, K, 100 μm; C, F, H, 50 μm.
Figure 2.
Interneurons in cortex are generated independently of cortical astrocytes. Approximately 5% of adult TFC.09 × Z/EG mice exhibited widely distributed GFP+ cells within cortex that were not arranged in columns (A). In these mice, GFP+ cells were distributed sparsely in layers 2 through 6 of neocortex through the entire anteroposterior and mediolateral axes (A, B). The GFP+ cells in cortex of these mice displayed the characteristic morphologies of interneurons, with fine local processes (C). The cortices of these mice did not contain labeled astrocytes (C). TFC.09 × Z/EG mice with interneurons in cortex always had GFP+ cells broadly distributed through their ipsilateral striatum (A, B, D). In mice with GFP+ interneurons in cortex, the striatum contained both GFP+ neurons and astrocytes (D). In the striata of these mice, GFP+ neurons and astrocytes had the characteristic morphologies of medium spiny interneurons and protoplasmic astrocytes, respectively (D). C and D are high-magnification views of the insets labeled “C” and “D” in B. B is a higher-magnification view of the inset in A. Scale bars: A, 500 μm; B, 250 μm; C, D, 50 μm.
Figure 3.
Non-neuronal GFP+ cells displayed characteristics of protoplasmic astrocytes. A–C, Non-neuronal GFP+ cells in developmental columns in adult mice exhibited characteristic protoplasmic astrocyte morphologies (arrows). D–F, A total of 97.5 ± 1.5% (603 cells examined in n = 3 mice) of non-neuronal GFP+ cells expressed S100B, an astrocytic marker. A total of 8.2 ± 3.7% of non-neuronal GFP+ cells (611 cells examined in n = 3 mice) expressed GFAP, a protein expressed in reactive and fibrous astrocytes in cortex. G–I, Labeled cells with neuronal morphologies expressed neither S100B nor GFAP (arrowheads). Conversely, GFP+ cells with astrocytic morphologies did not express NeuN, a mature neuronal marker. The processes of GFP+ astrocytes often encompassed GFP+ neurons. Scale bars: A–F, 10 μm; G–I, 25 μm.
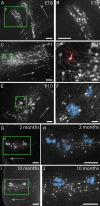
Figure 4.
Astrocytes develop within cortical columns and maintain their spatial relationship through adulthood. A, B, At E18, columns of radially arrayed projection neurons were visible in cortex. C, D, By P1, GFP+ cells with morphologies resembling immature astrocytes (pseudocolored in red) were visible within the columns. E, F, At P10, mature astrocytes (pseudocolored in blue in F) were found within columns distributed through the depth of cortex, much like they are in adult mice. G, H, Columns in 2-month-old adult mice contained pyramidal neurons and multiple clusters of multiple astrocytes. GFP+ astrocytes were still found closely arrayed with columns of GFP+ cortical neurons in 10-month-old mice, indicating that astrocyte addition and/or turnover in the adult mouse does not appear to result in the widespread dispersion of astrocytes. Scale bars: A–C, E, G–J, 100 μm; D, F; 25 μm. Mature astrocytes are pseudocolored in blue in E, H, and J. An immature astrocyte is pseudocolored in red in D. Arrows in A, C, E, G, and I point toward the pial surface of the neocortex.
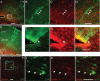
Figure 5.
GFP+ cells express radial glia markers zebrin and nestin during development. Between E16 and E18, GFP+ cells form columns of extending from the ventricles to the pia. GFP+ cells (green) near the ventricles in these columns expressed Nestin (A–D, red) or Zebrin (E–H, red), proteins expressed by radial glia. By P0, zebrin+/GFP+ cells were found in the parenchyma of cortex, away from the ventricular wall, presenting morphologies resembling a shortened radial glia. A–D, Arrows indicate GFP+/Nestin+ cells. E–L, Arrows indicate GFP+/zebrin+ processes and cells. A, E, F, Asterisks indicate the neocortical ventricular wall. Zebrin+/GFP+ cells were absent by P4, a time by which most radial glia have disappeared from the brain. Scale bars, 50 μm.
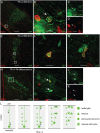
Figure 6.
Astrocyte precursors proliferate within cortical columns. To identify proliferating cells, mice were treated with BrdU and killed 4 h later. A–D, At P0, dividing, BrdU+ (red), GFP+ (green) cells were most frequently found at the bases of cortical columns, nearest the ventricle. E–G, By P2, the majority of dividing GFP+ cells were distributed within cortex and few were found near the bases of columns. To examine the fate of dividing GFP+ cells, we treated mice with BrdU at P0 and killed them at P4. GFP+ cells labeled with BrdU at P0 were found through the depth of cortex. H–K, GFP+/BRDU+ cells were also often found in pairs, suggesting that they differentiated near the site of their final division. These results suggest the following model of cortical astrogenesis (L): Radial glia first generate columns of projection neurons in cortex. Subsequently, the radial glia generate several astrocytic precursors that distribute radially along the column of neurons. These precursors undergo their final divisions in the different layers of the cortex, forming multiple clusters of multiple astrocytes within a developmental cortical column. Scale bars: A, E, H, 100 μm; B–D, F, G, I–K, 10 μm.
Similar articles
- Mature astrocytes transform into transitional radial glia within adult mouse neocortex that supports directed migration of transplanted immature neurons.
Leavitt BR, Hernit-Grant CS, Macklis JD. Leavitt BR, et al. Exp Neurol. 1999 May;157(1):43-57. doi: 10.1006/exnr.1999.6982. Exp Neurol. 1999. PMID: 10222107 - Fate mapping by piggyBac transposase reveals that neocortical GLAST+ progenitors generate more astrocytes than Nestin+ progenitors in rat neocortex.
Siddiqi F, Chen F, Aron AW, Fiondella CG, Patel K, LoTurco JJ. Siddiqi F, et al. Cereb Cortex. 2014 Feb;24(2):508-20. doi: 10.1093/cercor/bhs332. Epub 2012 Oct 31. Cereb Cortex. 2014. PMID: 23118195 Free PMC article. - Constructing circuits: neurogenesis and migration in the developing neocortex.
Kriegstein AR. Kriegstein AR. Epilepsia. 2005;46 Suppl 7:15-21. doi: 10.1111/j.1528-1167.2005.00304.x. Epilepsia. 2005. PMID: 16201991 - Mechanisms that regulate the number of neurons during mouse neocortical development.
Miyata T, Kawaguchi D, Kawaguchi A, Gotoh Y. Miyata T, et al. Curr Opin Neurobiol. 2010 Feb;20(1):22-8. doi: 10.1016/j.conb.2010.01.001. Curr Opin Neurobiol. 2010. PMID: 20138502 Review. - Transcriptional co-regulation of neuronal migration and laminar identity in the neocortex.
Kwan KY, Sestan N, Anton ES. Kwan KY, et al. Development. 2012 May;139(9):1535-46. doi: 10.1242/dev.069963. Development. 2012. PMID: 22492350 Free PMC article. Review.
Cited by
- Cortical astrocytes develop in a plastic manner at both clonal and cellular levels.
Clavreul S, Abdeladim L, Hernández-Garzón E, Niculescu D, Durand J, Ieng SH, Barry R, Bonvento G, Beaurepaire E, Livet J, Loulier K. Clavreul S, et al. Nat Commun. 2019 Oct 25;10(1):4884. doi: 10.1038/s41467-019-12791-5. Nat Commun. 2019. PMID: 31653848 Free PMC article. - Temporal-spatial Generation of Astrocytes in the Developing Diencephalon.
Hong W, Gong P, Pan X, Ren Z, Liu Y, Qi G, Li JL, Sun W, Ge WP, Zhang CL, Duan S, Qin S. Hong W, et al. Neurosci Bull. 2024 Jan;40(1):1-16. doi: 10.1007/s12264-023-01131-9. Epub 2023 Oct 16. Neurosci Bull. 2024. PMID: 37843774 Free PMC article. - Lineage-guided Notch-dependent gliogenesis by Drosophila multi-potent progenitors.
Ren Q, Awasaki T, Wang YC, Huang YF, Lee T. Ren Q, et al. Development. 2018 Jun 11;145(11):dev160127. doi: 10.1242/dev.160127. Development. 2018. PMID: 29764857 Free PMC article. - Switching modes in corticogenesis: mechanisms of neuronal subtype transitions and integration in the cerebral cortex.
Toma K, Hanashima C. Toma K, et al. Front Neurosci. 2015 Aug 11;9:274. doi: 10.3389/fnins.2015.00274. eCollection 2015. Front Neurosci. 2015. PMID: 26321900 Free PMC article. Review. - Multiple origins and modularity in the spatiotemporal emergence of cerebellar astrocyte heterogeneity.
Cerrato V, Parmigiani E, Figueres-Oñate M, Betizeau M, Aprato J, Nanavaty I, Berchialla P, Luzzati F, de'Sperati C, López-Mascaraque L, Buffo A. Cerrato V, et al. PLoS Biol. 2018 Sep 27;16(9):e2005513. doi: 10.1371/journal.pbio.2005513. eCollection 2018 Sep. PLoS Biol. 2018. PMID: 30260948 Free PMC article.
References
- Allen NJ, Barres BA. Signaling between glia and neurons: focus on synaptic plasticity. Curr Opin Neurobiol. 2005;15:542–548. - PubMed
- Allen NJ, Barres BA. Neuroscience: Glia—more than just brain glue. Nature. 2009;457:675–677. - PubMed
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–890. - PubMed
- Costa MR, Bucholz O, Schroeder T, Götz M. Late origin of glia-restricted progenitors in the developing mouse cerebral cortex. Cereb Cortex. 2009;19(Suppl 1):i135. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases