Reducing human apolipoprotein E levels attenuates age-dependent Aβ accumulation in mutant human amyloid precursor protein transgenic mice - PubMed (original) (raw)
Comparative Study
Reducing human apolipoprotein E levels attenuates age-dependent Aβ accumulation in mutant human amyloid precursor protein transgenic mice
Nga Bien-Ly et al. J Neurosci. 2012.
Abstract
Apolipoprotein E4 (apoE4) plays a major role in the pathogenesis of Alzheimer's disease. Brain amyloid-β (Aβ) accumulation depends on age and apoE isoforms (apoE4 > apoE3) both in humans and in transgenic mouse models. Brain apoE levels are also isoform dependent, but in the opposite direction (apoE4 < apoE3). Thus, one prevailing hypothesis is to increase brain apoE expression to reduce Aβ levels. To test this hypothesis, we generated mutant human amyloid precursor protein transgenic mice expressing one or two copies of the human APOE3 or APOE4 gene that was knocked in and flanked by LoxP sites. We report that reducing apoE3 or apoE4 expression by 50% in 6-month-old mice results in efficient Aβ clearance and does not increase Aβ accumulation. However, 12-month-old mice with one copy of the human APOE gene had significantly reduced Aβ levels and plaque loads compared with mice with two copies, regardless of which human apoE isoform was expressed, suggesting a gene dose-dependent effect of apoE on Aβ accumulation in aged mice. Additionally, 12-month-old mice expressing one or two copies of the human APOE4 gene had significantly higher levels of Aβ accumulation and plaque loads than age-matched mice expressing one or two copies of the human APOE3 gene, suggesting an isoform-dependent effect of apoE on Aβ accumulation in aged mice. Moreover, Cre-mediated APOE4 gene excision in hippocampal astrocytes significantly reduced insoluble Aβ in adult mice. Thus, reducing, rather than increasing, apoE expression is an attractive approach to lowering brain Aβ levels.
Figures
Figure 1.
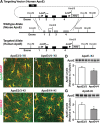
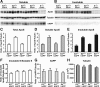
Generation and characterization of floxed human apoE knock-in mice. A, Targeting vector containing human apoE exons 2–4 (represented by black rectangles) flanked by loxP sites with endogenous mouse exon 1 and mouse upstream and downstream elements used for homologous recombination (mouse exons represented by gray rectangles). B, C, E, F, Representative immunofluorescence images for apoE (green) and GFAP (red) in the hippocampal subfield highlighting apoE-producing astrocytes in APOE-fKI mice (B, C) and APOE-KI mice (E, F). D, G, Western blot and densitometric quantification of apoE protein levels in the hippocampus of homozygous APOE-fKI mice (D) and homozygous APOE-KI mice (G). N = 3–4 mice per genotype. Values are mean ± SEM. *p < 0.05 by two-tailed, unpaired t test. Scale bar, 10 μm.
Figure 2.
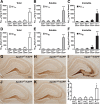
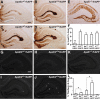
Reducing apoE levels by half does not increase Aβ accumulation in young mice. A–C, Levels of Aβ1-x were measured by sandwich ELISA in combined soluble and insoluble hippocampal lysates (A), low-detergent-soluble lysates (B), and insoluble lysates (C) for E3+/−/hAPP, E3+/+/hAPP, E4+/−/hAPP, E4+/+/hAPP, and E−/−/hAPP mice at 6–8 months of age. D–F, Levels of Aβ42 in combined soluble and insoluble lysates (D), low-detergent-soluble lysates (E), and insoluble lysates (F). G–K, Representative sections from an Aβ immunostain of the hippocampal subfield of E3+/−/hAPP (G), E3+/+/hAPP (H), E4+/−/hAPP (J), E4+/+/hAPP (K), and E−/−/hAPP (I) mice. L, Quantification by densitometry of the percentage area covered by Aβ deposition. N = 7–12 mice per genotype, three sections per mouse. Values are mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 versus all other groups by one-way ANOVA with Bonferroni's post hoc test. Scale bar, 250 μm.
Figure 3.
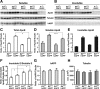
Human apoE expression levels are reduced in young hemizygous apoE mice without altering APP expression levels. A, B, Western blotting was performed on low-detergent-soluble (A) and insoluble (B) hippocampal lysates and probed for apoE, tubulin, and human full-length APP protein levels in E3+/−/hAPP, E3+/+/hAPP, E4+/−/hAPP, E4+/+/hAPP, and E−/−/hAPP mice at 6–8 months of age. C–F, Quantification of Western blots by densitometry for total apoE levels (C), low-detergent-soluble apoE (D), insoluble apoE (E), and ratios of soluble to insoluble pools of apoE (F). G, H, Western blot quantification of total full-length APP levels (G) and total tubulin levels as loading controls (H). Values are mean ± SEM. N = 3–4 mice per genotype. *p < 0.05, ***p < 0.001, by two-tailed, unpaired t test.
Figure 4.
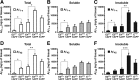
Halving the levels of apoE significantly attenuates Aβ accumulation in aged mice. A–F, Levels of Aβ1-x (A–C) and Aβ42 (D–F) were measured by sandwich ELISA in both soluble and insoluble lysates (A, D), low-detergent-soluble lysates (B, E), and insoluble lysates (C, F) in the hippocampus of E3+/−/hAPP, E3+/+/hAPP, E4+/−/hAPP, E4+/+/hAPP, and E−/−/hAPP mice at 12 months of age. Values are mean ± SEM. N = 6–13 mice per genotype. *p < 0.05, **p < 0.01, and ***p < 0.001, by two-tailed, unpaired t test.
Figure 5.
Human apoE expression levels are reduced in aged hemizygous apoE mice without altering APP expression levels. A, B, Western blotting was performed on low-detergent-soluble (A) and insoluble (B) hippocampal lysates, and probed for apoE, tubulin, and human full-length APP protein levels in E3+/−/hAPP, E3+/+/hAPP, E4+/−/hAPP, E4+/+/hAPP, and E−/−/hAPP mice at 12 months of age. C–F, Quantification of Western blots by densitometry for total apoE levels (C), low-detergent-soluble apoE (D), insoluble apoE (E), and ratios of soluble to insoluble pools of apoE (F). G, H, Western blot quantification of total full-length APP levels (G) and total tubulin levels as loading controls (H). Values are mean ± SEM. N = 3–4 mice per genotype.
Figure 6.
Halving the levels of apoE significantly attenuates Aβ deposition in aged mice. A–E, Representative sections from 12-month-old aged E3+/−/hAPP (A), E3+/+/hAPP (B), E4+/−/hAPP (C), E4+/+/hAPP (D), and E−/−/APP (E) mice immunostained for Aβ. F, Quantification of Aβ immunostain by densitometry for the percentage area of Aβ deposition in the five genotypes of mice. G–K, Representative sections from 12-month-old E3+/−/hAPP (G), E3+/+/hAPP (H), E4+/−/hAPP (I), E4+/+/hAPP (J), and E−/−/hAPP (K) mice stained with thioflavin S dye to fluorescently label fibrillar/amyloid plaques. L, Quantification of percentage area positive for thioflavin S by densitometry. Values are mean ± SEM. N = 6–13 mice per genotype, three sections per mouse. *p < 0.05, by two-tailed, unpaired t test; **p < 0.01 versus all other groups by one-way ANOVA with Bonferroni's post hoc test. Scale bars, 250 μm.
Figure 7.
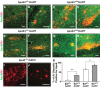
Gene dose- and isoform-dependent codistribution of apoE with Aβ plaques in aged mice. A–J, Representative fluorescent immunostaining for apoE (green) and Aβ (red) in various subregions of the hippocampus in E3+/−/hAPP (A, B), E3+/+/hAPP (C, D), E4+/−/hAPP (E, F), E4+/+/hAPP (G, H), and E−/−/hAPP (I, J) mice at 12 months of age. K, Quantification of Aβ deposits and codistribution with apoE. Mice with two copies of apoE3 or apoE4 have a greater percentage of plaques containing apoE. Values are mean ± SEM. N = 3–4 mice per genotype, two sections per mouse. *p < 0.05, **p < 0.01, by two-tailed, unpaired t test. Scale bar, 25 μm.
Figure 8.
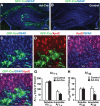
Cre-mediated APOE gene excision in hippocampal astrocytes decreases Aβ levels in young adult mice. A, B, Representative confocal images of brain sections from E4+/+/hAPP mice at 6–7 months of age stained for GFAP (blue) from the hippocampus injected with adenovirus expressing Cre (Ad-Cre) and GFP (green) (A) or saline (B). Brains were collected 1 month after the Ad-Cre virus or saline injection. C–F, Representative confocal images of brain sections from E4+/+/hAPP mice stained for GFP (green; C, D, F), GFAP (blue; C, E, F), and apoE (red; D–F) from the hippocampus injected with Ad-Cre and expressing GFP (green). G, H, Aβ levels in Ad-Cre-injected and saline-injected hippocampi were quantified 1 month after injection. Levels of Aβ1-x (G) in both soluble and insoluble hippocampal lysates were similar in control compared with Ad-Cre-injected hemibrains, whereas levels of Aβ42 (H) showed a specific difference in the insoluble lysates. Values are mean ± SEM. N = 8 mice. *p < 0.05 by two-tailed, paired t test. Scale bars: A, B, 250 μm; C–F, 20 μm.
Similar articles
- Accumulation of amyloid-β in the brain of mouse models of Alzheimer's disease is modified by altered gene expression in the presence of human apoE isoforms during aging.
Honda K, Saito Y, Saito H, Toyoda M, Abe R, Saito T, Saido TC, Michikawa M, Taru H, Sobu Y, Hata S, Nakaya T, Suzuki T. Honda K, et al. Neurobiol Aging. 2023 Mar;123:63-74. doi: 10.1016/j.neurobiolaging.2022.12.003. Epub 2022 Dec 17. Neurobiol Aging. 2023. PMID: 36638682 - Modulation of Alzheimer-like synaptic and cholinergic deficits in transgenic mice by human apolipoprotein E depends on isoform, aging, and overexpression of amyloid beta peptides but not on plaque formation.
Buttini M, Yu GQ, Shockley K, Huang Y, Jones B, Masliah E, Mallory M, Yeo T, Longo FM, Mucke L. Buttini M, et al. J Neurosci. 2002 Dec 15;22(24):10539-48. doi: 10.1523/JNEUROSCI.22-24-10539.2002. J Neurosci. 2002. PMID: 12486146 Free PMC article. - Selective reduction of astrocyte apoE3 and apoE4 strongly reduces Aβ accumulation and plaque-related pathology in a mouse model of amyloidosis.
Mahan TE, Wang C, Bao X, Choudhury A, Ulrich JD, Holtzman DM. Mahan TE, et al. Mol Neurodegener. 2022 Feb 2;17(1):13. doi: 10.1186/s13024-022-00516-0. Mol Neurodegener. 2022. PMID: 35109920 Free PMC article. - Apolipoprotein E isoforms in Alzheimer's disease pathology and etiology.
Baum L, Chen L, Ng HK, Pang CP. Baum L, et al. Microsc Res Tech. 2000 Aug 15;50(4):278-81. doi: 10.1002/1097-0029(20000815)50:4<278::AID-JEMT5>3.0.CO;2-T. Microsc Res Tech. 2000. PMID: 10936880 Review. - Apolipoprotein E and oxidative stress in brain with relevance to Alzheimer's disease.
Butterfield DA, Mattson MP. Butterfield DA, et al. Neurobiol Dis. 2020 May;138:104795. doi: 10.1016/j.nbd.2020.104795. Epub 2020 Feb 6. Neurobiol Dis. 2020. PMID: 32036033 Free PMC article. Review.
Cited by
- Age-dependent effects of APOE ε4 in preclinical Alzheimer's disease.
Bonham LW, Geier EG, Fan CC, Leong JK, Besser L, Kukull WA, Kornak J, Andreassen OA, Schellenberg GD, Rosen HJ, Dillon WP, Hess CP, Miller BL, Dale AM, Desikan RS, Yokoyama JS. Bonham LW, et al. Ann Clin Transl Neurol. 2016 Aug 26;3(9):668-77. doi: 10.1002/acn3.333. eCollection 2016 Sep. Ann Clin Transl Neurol. 2016. PMID: 27648456 Free PMC article. - Anti-apoE immunotherapy inhibits amyloid accumulation in a transgenic mouse model of Aβ amyloidosis.
Kim J, Eltorai AE, Jiang H, Liao F, Verghese PB, Kim J, Stewart FR, Basak JM, Holtzman DM. Kim J, et al. J Exp Med. 2012 Nov 19;209(12):2149-56. doi: 10.1084/jem.20121274. Epub 2012 Nov 5. J Exp Med. 2012. PMID: 23129750 Free PMC article. - Experimental and real-world evidence supporting the computational repurposing of bumetanide for _APOE4_-related Alzheimer's disease.
Taubes A, Nova P, Zalocusky KA, Kosti I, Bicak M, Zilberter MY, Hao Y, Yoon SY, Oskotsky T, Pineda S, Chen B, Jones EAA, Choudhary K, Grone B, Balestra ME, Chaudhry F, Paranjpe I, De Freitas J, Koutsodendris N, Chen N, Wang C, Chang W, An A, Glicksberg BS, Sirota M, Huang Y. Taubes A, et al. Nat Aging. 2021 Oct;1(10):932-947. doi: 10.1038/s43587-021-00122-7. Epub 2021 Oct 11. Nat Aging. 2021. PMID: 36172600 Free PMC article. - Linking Cerebral Malaria Pathogenesis to APOE-Mediated Amyloidosis: Observations and Hypothesis.
Kioko M, Mwangi S, Njunge JM, Berkley JA, Bejon P, Abdi AI. Kioko M, et al. Mol Neurobiol. 2024 Jul 18. doi: 10.1007/s12035-024-04366-3. Online ahead of print. Mol Neurobiol. 2024. PMID: 39023792 Review. - ApoE4 reduction: An emerging and promising therapeutic strategy for Alzheimer's disease.
Li Y, Macyczko JR, Liu CC, Bu G. Li Y, et al. Neurobiol Aging. 2022 Jul;115:20-28. doi: 10.1016/j.neurobiolaging.2022.03.011. Epub 2022 Mar 22. Neurobiol Aging. 2022. PMID: 35453035 Free PMC article. Review.
References
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. - PubMed
- Bertrand P, Poirier J, Oda T, Finch CE, Pasinetti GM. Association of apolipoprotein E genotype with brain levels of apolipoprotein E and apolipoprotein J (clusterin) in Alzheimer's disease. Brain Res Mol Brain Res. 1995;33:174–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous