Negative regulation of miR-145 by C/EBP-β through the Akt pathway in cancer cells - PubMed (original) (raw)
Negative regulation of miR-145 by C/EBP-β through the Akt pathway in cancer cells
Mohit Sachdeva et al. Nucleic Acids Res. 2012 Aug.
Abstract
MicroRNAs are master gene regulators that can also be under the control of transcriptional regulation. We have previously shown that miR-145 is a tumor suppressor capable of silencing c-Myc and the tumor suppressor p53 induces miR-145 by directly binding to the miR-145 promoter, demonstrating the role of miR-145 in p53-mediated c-Myc repression. However, little is known as to why miR-145 is often downregulated in tumors. In this study, we identify CCAAT/enhancer binding protein beta (C/EBP-β) as a negative regulator for miR-145 expression by direct interaction with the putative C/EBP-β binding site in the miR-145 promoter. In the wild-type p53 background, C/EBP-β counteracts the ability of p53 to induce miR-145. Moreover, C/EBP-β is able to suppress miR-145 in the mutant p53 background, suggesting the p53 independent regulation of miR-145. Of interest, both the large isoform (LAP-2) and the small isoform (LIP) of C/EBP-β can exert suppressive function for miR-145. Finally, we further show that, like serum starvation and PI3K inhibitor LY29, the antioxidant resveratrol suppresses pAkt and phosphorylation of C/EBP-β and at the same time, it induces miR-145. Together, these results suggest a miR-145 regulatory system involving the Akt and C/EBP-β, which may contribute to the downregulation of miR-145 in cancer cells.
Figures
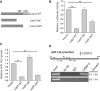
Figure 1.
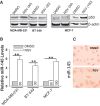
Induction of miR-145 by resveratrol (RSV). (A) Expression of p53 in MCF-10A, 231 and BT-549, MCF-7 and T47D by western. (B) RSV at 30 µM induced miR-145 in MDA-MB-231 and BT-549 cells, respectively. However, in MCF-7 cells the same concentration of RSV did not induce miR-145 until the concentration was increased to 100 µM. Cells were treated with RSV for 24 h before RNA extraction, followed by real time RT–PCR. (C) In situ hybridization also detected an increase of miR-145 after RSV treatment (30 µM) in MDA-MB-231 cells. Values in (B) are mean ± SE, n = 3. **P < 0.01 compared with vector control.
Figure 2.
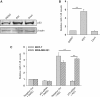
Induction of miR-145 in a p53 independent manner by RSV. (A) Expression of p53 in response to RSV and doxo. MDA-MB-231 cells were treated with either RSV (30 µM) or doxo (1 µg/ml) for 24 h and then harvested for protein extraction and western. (B) Detection of miR-145 by real-time RT–PCR after treatment of RSV or doxo. The same treatment was done as in (A) before extraction of total RNA. (C) Effect of suppression of p53 on the RSV-mediated induction of miR-145 as detected by real-time RT–PCR. Cells were first transfected with control siRNA (Ctrl) or p53-siRNA and were subsequently treated with RSV (30 µM for MDA-MB-231; 100 µM for MCF-7). RNA was extracted 24 h after RSV treatment. Values in (B) and (C) are mean ± SE, n = 3. **P < 0.01 compared with vector control.
Figure 3.
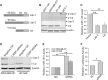
Identification of C/EBP-β as a potential negative regulator of miR-145. Experiments were performed in transfected 293T cells. (A) Schematic description of the putative miR-145 promoter with potential transcription factor binding sites and pre-miR-145 start site (an arrow). (B) Luciferase assays using pMir-145p-Luc reporter. (C) GFP expression using pMir-145p-GFP. (D) Effect of C/EBP-β on miR-145, as detected by real-time RT–PCR. Values in (B) and (D) are mean ± SE, n = 3. **P < 0.01 compared with vector control.
Figure 4.
Both Lap-2 and Lip isoforms of C/EBP-β suppress the endogenous miR-145 and the miR-145 promoter activity. (A) Schematic description of Lap-1, Lap-2 and Lip with their predicted molecular weights. DB: DNA binding domain; DD: dimerization domain. (B) Expression of C/EBP-β isoforms in various cell lines. Note that only Lap-2 and Lip were detectable in these cells by western. (C) Effect of Lap-2 and Lip on miR-145. MDA-MB-231 cells were infected with vector alone or Lap or Lip expression vector. RNA from these cells was extracted and real-time RT–PCR was performed as described in ‘Materials and Methods’ section. (D) Suppression of Lap-2 and Lip by C/EBP-β-siRNAs in MDA-MB-231 and BT-549 cells. Cells were transfected with negative control (Ctrl) or C/EBP-β siRNAs as described in ‘Materials and Methods’ section; 24 h after transfection, the cells were harvested for western. (E) Effect of C/EBP-β siRNAs on miR-145 as detected by real-time RT–PCR. The same cells as in (D) were used for RNA extraction. (F) Effect of C/EBP-β siRNAs on promoter activity. 293T cells were first transfected with the miR-145 promoter luciferase reporter and subsequently with C/EBP-β siRNAs. The cells were extracted for luciferase assays 24 h after transfection. Values in (C), (E) and (F) are mean ± SE, n = 3. **P < 0.01; *P < 0.05 compared with vector control.
Figure 5.
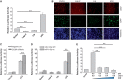
Effect of C/EBP-β on the p53-mediated induction of miR-145 promoter activity. (A) While miR-145 promoter luciferase activity is suppressed by Lap-2 and Lip, p53 induces the luciferase activity. The 293T cells were co-transfected with both the luciferase reporter (pMir-145p-Luc) along with Lap-2 or Lip, or p53 expression vector. Luciferase assays were carried out 24 after transfection. (B) Detection of the effect of C/EBP-β and p53 on the miR-145 promoter activity by the GFP reporter (pMir-145p-GFP). The 293T cells were transfected with pMir-145p-GFP along with Lap-2, Lip or p53 and were examined under a fluorescence microscope 24 h after transfection. The RFP carrying vector was used to ectopically express Lap-2, Lip and p53, respectively. GFP pictures were taken at the same exposure time for all of them. Decreases or increases in GFP intensity represent reductions or inductions of miR-145 promoter activity. (C) Effect of C/EBP-β siRNAs on luciferase activity combined with ectopic expression of p53. 293T cells were co-transfected with the luciferase reporter and p53 expression vector, and subsequently transfected with negative control or C/EBP-β siRNAs. Luciferase assays were performed 24 h after transfection of siRNAs. (D) The C/EBP-β binding site of the miR-145 promoter is important for C/EBP-β to suppress miR-145. The 293T cells were co-transfected with a luciferase reporter (miR-145p-WT or miR-145p-m) along with expression vectors as indicated. Luciferase assays were performed 24 h after transfection. (E) Lap-2 represses p53-mediated induction of miR-145 promoter activity. The 293T cells were co-transfected with the miR-145 promoter luciferase reporter and p53 expression vector along with various amounts of Lap-2 expression vector. Values in (A), (C), (D) and (E) are mean ± SE, n = 3. **P < 0.01 compared with vector control.
Figure 6.
Suppression of miR-145 by Lap-2 involves DNA binding domain. (A) Schematic description of domain structure of C/EBP-β. DB: DNA binding domain; DD: dimerization domain. (B) Effect of Lap-2 deletion on the miR-145 promoter luciferase activity. The 293T cells were co-transfected with the miR-145 promoter luciferase reporter and Lap-2-WT or deleted Lap-2 expression vectors (Lap-2-del or Lap-2-del1). Luciferase assays were performed 24 h after transfection. (C) Effect of Lap-2 deletion on expression of the endogenous miR-145. MDA-MB-231 cells were infected with Lap-2-WT, Lap-2-del or Lap-2-del1. RNA was extracted from the infected cells for detection of miR-145 by real-time RT–PCR. (D) C/EBP-β directly interacts with the miR-145 promoter in MDA-MB-231 cells as detected by ChIP assays, following the previously described procedure (11). Values in (B) and (C) are mean ± SE, n = 3. **P < 0.01 compared with vector control.
Figure 7.
Suppression of phosphorylated Akt (p-Akt) and phosphorylated Lap-2 (p-Lap-2) by RSV. (A) Expression of Akt and pAkt in MDA-MB-231, BT-549 and MCF-7 cells. (B) RSV suppresses both p-Akt and p-C/EBP-β in MDA-MB-231, BT-549 and MCF-7 cells. (C) Induction of p-C/EBP-β by RSV in MDA-MB-231 cells as detected by ICC. Cells were treated with RSV at 30 µM for 24 h and then were subject to ICC analysis. (D) RSV suppresses the miR-145 target c-Myc in MDA-MB-231 and BT-549 cells, which is associated with suppression of p-Lap-2. (E) Both RSV and LY29 reduce the binding of C/EBP-β to the miR-145 promoter in MDA-MB-231 cells as detected by ChIP assays. Note that the PCR band in C/EBP-β lane was decreased in both RVS and LY29 treatment, compared with DMSO.
Similar articles
- p53 represses c-Myc through induction of the tumor suppressor miR-145.
Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. Sachdeva M, et al. Proc Natl Acad Sci U S A. 2009 Mar 3;106(9):3207-12. doi: 10.1073/pnas.0808042106. Epub 2009 Feb 6. Proc Natl Acad Sci U S A. 2009. PMID: 19202062 Free PMC article. - Transcription factor C/EBP-β induces tumor-suppressor phosphatase PHLPP2 through repression of the miR-17-92 cluster in differentiating AML cells.
Yan Y, Hanse EA, Stedman K, Benson JM, Lowman XH, Subramanian S, Kelekar A. Yan Y, et al. Cell Death Differ. 2016 Jul;23(7):1232-42. doi: 10.1038/cdd.2016.1. Epub 2016 Feb 12. Cell Death Differ. 2016. PMID: 26868909 Free PMC article. - Stat3 and CCAAT/enhancer binding protein beta (C/EBP-beta) regulate Jab1/CSN5 expression in mammary carcinoma cells.
Shackleford TJ, Zhang Q, Tian L, Vu TT, Korapati AL, Baumgartner AM, Le XF, Liao WS, Claret FX. Shackleford TJ, et al. Breast Cancer Res. 2011 Jun 20;13(3):R65. doi: 10.1186/bcr2902. Breast Cancer Res. 2011. PMID: 21689417 Free PMC article. - VPS33B interacts with NESG1 to modulate EGFR/PI3K/AKT/c-Myc/P53/miR-133a-3p signaling and induce 5-fluorouracil sensitivity in nasopharyngeal carcinoma.
Liang Z, Liu Z, Cheng C, Wang H, Deng X, Liu J, Liu C, Li Y, Fang W. Liang Z, et al. Cell Death Dis. 2019 Apr 3;10(4):305. doi: 10.1038/s41419-019-1457-9. Cell Death Dis. 2019. PMID: 30944308 Free PMC article. - C/EBPß Isoform Specific Gene Regulation: It's a Lot more Complicated than you Think!
Spike AJ, Rosen JM. Spike AJ, et al. J Mammary Gland Biol Neoplasia. 2020 Mar;25(1):1-12. doi: 10.1007/s10911-020-09444-5. Epub 2020 Feb 20. J Mammary Gland Biol Neoplasia. 2020. PMID: 32078094 Free PMC article. Review.
Cited by
- Modulation of microRNAs by phytochemicals in cancer: underlying mechanisms and translational significance.
Srivastava SK, Arora S, Averett C, Singh S, Singh AP. Srivastava SK, et al. Biomed Res Int. 2015;2015:848710. doi: 10.1155/2015/848710. Epub 2015 Mar 17. Biomed Res Int. 2015. PMID: 25853141 Free PMC article. Review. - C/EBP-β-activated microRNA-223 promotes tumour growth through targeting RASA1 in human colorectal cancer.
Sun D, Wang C, Long S, Ma Y, Guo Y, Huang Z, Chen X, Zhang C, Chen J, Zhang J. Sun D, et al. Br J Cancer. 2015 Apr 28;112(9):1491-500. doi: 10.1038/bjc.2015.107. Epub 2015 Mar 31. Br J Cancer. 2015. PMID: 25867276 Free PMC article. - Assessment of biochemical recurrence of prostate cancer (Review).
Lin X, Kapoor A, Gu Y, Chow MJ, Xu H, Major P, Tang D. Lin X, et al. Int J Oncol. 2019 Dec;55(6):1194-1212. doi: 10.3892/ijo.2019.4893. Epub 2019 Oct 4. Int J Oncol. 2019. PMID: 31638194 Free PMC article. - MiR-145 inhibits tumor angiogenesis and growth by N-RAS and VEGF.
Zou C, Xu Q, Mao F, Li D, Bian C, Liu LZ, Jiang Y, Chen X, Qi Y, Zhang X, Wang X, Sun Q, Kung HF, Lin MC, Dress A, Wardle F, Jiang BH, Lai L. Zou C, et al. Cell Cycle. 2012 Jun 1;11(11):2137-45. doi: 10.4161/cc.20598. Epub 2012 Jun 1. Cell Cycle. 2012. PMID: 22592534 Free PMC article. - Polyphenol-Enriched Blueberry Preparation Controls Breast Cancer Stem Cells by Targeting FOXO1 and miR-145.
Mallet JF, Shahbazi R, Alsadi N, Matar C. Mallet JF, et al. Molecules. 2021 Jul 17;26(14):4330. doi: 10.3390/molecules26144330. Molecules. 2021. PMID: 34299605 Free PMC article.
References
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat. Rev. Cancer. 2006;6:857–866. - PubMed
- Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. - PubMed
- Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) J. Biol. Chem. 2007;282:14328–14336. - PubMed
- Si ML, Zhu S, Wu H, Lu Z, Wu F, Mo YY. miR-21-mediated tumor growth. Oncogene. 2007;26:2799–2803. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous