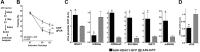HDAC1 regulates fear extinction in mice - PubMed (original) (raw)
HDAC1 regulates fear extinction in mice
Sanaz Bahari-Javan et al. J Neurosci. 2012.
Abstract
Histone acetylation has been implicated with the pathogenesis of neuropsychiatric disorders and targeting histone deacetylases (HDACs) using HDAC inhibitors was shown to be neuroprotective and to initiate neuroregenerative processes. However, little is known about the role of individual HDAC proteins during the pathogenesis of brain diseases. HDAC1 was found to be upregulated in patients suffering from neuropsychiatric diseases. Here, we show that virus-mediated overexpression of neuronal HDAC1 in the adult mouse hippocampus specifically affects the extinction of contextual fear memories, while other cognitive abilities were unaffected. In subsequent experiments we show that under physiological conditions, hippocampal HDAC1 is required for extinction learning via a mechanism that involves H3K9 deacetylation and subsequent trimethylation of target genes. In conclusion, our data show that hippocampal HDAC1 has a specific role in memory function.
Figures
Figure 1.
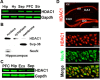
HDAC1 expression in the adult hippocampus. A, HDAC1 protein levels were analyzed in different brain areas by immunoblotting. B, Representative immunoblot images showing that hippocampal HDAC1 is highly enriched in the nuclear fraction. C, Representative immunoblot analysis showing HDAC1 protein in the postmortem human brain obtained from patients that did not suffer from any neuropsychiatric disorder. The image depicts the representative pattern observed in 4 individuals. For immunoblot analysis (A–C), 30 μg of protein were subjected to SDS-PAGE. NeuN, Neuronal Nuclei. D, Representative confocal images showing HDAC1 protein levels in the NeuN-positive neurons of the mouse hippocampus. Scale bars: Top, 200 μm; bottom, 20 μm. Ecx, Entorhinal cortex; Hip, hippocampus; Hy, hypothalamus; Pfc, prefrontal cortex; Sep, septum; Str, striatum; CA1, CA2, CA3, hippocampal subfields; cc, corpus callosum;, DG, dentate gyrus; py, pyramidal cell layer. Error bars indicate SEM.
Figure 2.
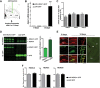
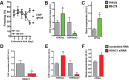
Overexpression of HDAC1 in the adult hippocampus. A, Experimental design. B, Quantitative real time PCR showed a sixfold increase of Hdac1 mRNA in the AVV-HDAC1-GFP-injected mice 14 d after injection (n = 3/group; ***p < 0.0001). C, The expression of other class I HDACs was unchanged in AAV-HDAC1-GFP-injected mice when analyzed 14 d after injection. D, Immunoblot analysis was performed 14 d after injection. When compared with endogenous HDAC1 levels, AAV-HDAC1-GFP-injected mice displayed a twofold increased HDAC1 levels (n = 4/group). E, Confocal imaging of hippocampal sections confirmed that nuclear HDAC1-GFP was detectable 14 but not 6 d after injection in AAV-HDAC1-GFP-treated mice (n = 4/group). Scale bar, 200 μm. At 14 d: Left panel displays colocalization of HDAC1 and HDAC1-GFP in the adult dorsal hippocampus. Middle panel displays corresponding high-magnification images. Right panel displays the ventral hippocampus where no HDAC1-GFP expression was detected. Scale bar, 50 μm. F, The same lysates as described in D were used to analyze protein levels of HDAC2, HDAC3 and HDAC8. No difference was observed among groups. Protein (30 μg) was used for immunoblot analysis. Error bars indicate SEM.
Figure 3.
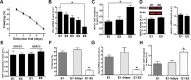
Cognitive function in AAV-HDAC1-GFP-treated mice. A, Left, Representative path of mice in a 5 min open field exposure. Right, Distance traveled during 5 min open field exposure. B, The ratio of time spent in the periphery vs the center of the open field was similar among groups. C, Depressive-like behavior was analyzed in the Porsolt forced swim test. No significant difference among groups was observed. D, Working memory as assessed by the cross-maze test was not affected in AAV-HDAC1-GFP-injected mice. E, Short-term memory (5 min) in the novel object recognition test was similar among groups. Dashed line indicates chance level. F, Long-term memory (24 h) was analyzed in the novel object recognition test. No difference among groups was observed. Dashed line indicates chance level. G, Associative memory was analyzed using contextual fear conditioning. Freezing was similar among groups. H, Left, The escape latency during water maze training was similar among groups. Right, No difference among groups was observed in the probe test performed 24 h after the last training session. I, Prepulse inhibition of the startle response was significantly increased at low intensities (70 dB) in AAV-HDAC1-GFP-treated mice (*p < 0.05 vs AAV-GFP). J, Startle response was not affected in AAV-HDAC1-GFP-injected animals. n = 9–10/group. Error bars indicate SEM.
Figure 4.
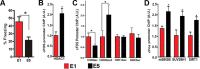
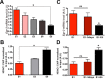
Hippocampal HDAC1 affects the extinction of fear memories. A, Top, Experimental design. Bottom, While AAV-HDAC1-GFP and AAV-GFP-injected mice (n = 10/group) show similar freezing behavior on E1, fear extinction learning was significantly enhanced in the AAV-HDAC1-GFP group (*p < 0.05, **p < 0.01 for day comparison; repeated measurements p = 0.001, F = 12,78_)_. B, Top, Experimental design. Bottom, When compared with the vehicle group (n = 21), intrahippocampal injection of MS-275 (n = 21) immediately after each extinction trial impaired fear extinction (*p < 0.05 for day comparison; repeated measurements p = 0.0038, F = 9,435). C, Intrahippocampal injection of MS-275 does not affect the consolidation of contextual fear memories. Microcannulae were implanted into the hippocampus of mice (n = 7/group). After recovery all animals were subjected to contextual fear conditioning. Immediately after the training mice were injected with the same concentration of MS-275 that was used for fear extinction experiments fear memory was analyzed 24 h later. Notably, freezing behavior during the memory test was similar between MS-275 and vehicle-injected mice. D, Left, Experimental design. Right, When compared with the control group that received a scrambled control RNA (n = 6), intrahippocampal injection of HDAC1 siRNA (n = 6) impaired fear extinction (*p < 0.05, **p < 0.001 for day comparison; repeated measurements p = 0.0001, F = 21,89). E, HDAC1 siRNA-treated mice display significantly reduced hippocampal levels of Hdac1 mRNA and HDAC1 protein, measured via qPCR and quantitative immunoblot respectively (n = 5/group)**p < 0.001 vs scrambled RNA). Error bars indicate SEM.
Figure 5.
Fear extinction-dependent recruitment of HDAC1 to the c-Fos promoter. A, Fear extinction in the mice (n = 45) used for molecular analysis in B-E (n = 5/group). B, c-Fos expression was analyzed via qPCR in hippocampal tissue isolated 1 h after exposure to extinction trials. The data are normalized to tissue obtained from a naive control group. C, HDAC1 ChIP was performed from hippocampal tissue 1 h after exposure to E1, E3, and E5. Note that the downregulation of c-Fos correlates with recruitment of HDAC1 to the c-Fos promoter. D, Normalized hippocampal HDAC1 protein levels (left, images show representative immunoblot analysis, 30 μg of hippocampal protein was loaded per lane) and mRNA levels (right) were similar among groups when compared 1 h after E1 and E5 exposure. E, ChIP analysis of the c-Fos promoter was performed for HDAC2 and HDAC3 after E1 and E5. No significant difference among groups was observed. F, Freezing behavior in the E1–3 d group is significantly higher when compared with the E1–E5 group (n = 5/group). G, c-Fos expression was measured 1 h after extinction trials. c-Fos levels were significantly higher in the E1–3 d group when compared with E1–E5 group (n = 5/group). H, HDAC1 ChIP was performed from hippocampal tissue 1 h after exposure to extinction trial in the E1, E1–3 d, and E1–E5 groups (n = 5/group). Note that the increased c-Fos expression in the E1–3 d group correlates with reduced HDAC1 level at the c-Fos promoter. Error bars indicate SEM.
Figure 6.
Chromatin remodeling at the c-Fos promoter during fear extinction. A, Mice (n = 10) were subjected to fear extinction training. Hippocampal tissue was isolated and prepared for ChIP analysis either 1 h after E1 or 1 h after E5 exposure. (*p < 0.05 E1 vs E5). B, ChIP analysis revealed significantly increased HDAC1 levels at the c-Fos promoter upon E5 exposure (p < 0.05 vs E1). C, ChIP analysis revealed decreased H3K9 acetylation and increased H3K9 trimethylation at the c-Fos promoter upon E5 exposure (*p < 0.05 vs E1). D, Increased mSIN3B, SUV39H1 and SIRT1 levels were detected at the c-Fos promoter upon E5 exposure (p < 0.05 vs E1). Error bars indicate SEM.
Figure 7.
HDAC1 regulates H3K9 modifications and c-Fos expression during fear extinction. A, Experimental design for B–D. B, Freezing behavior during E1 was indistinguishable between AAV-HDAC1-GFP and AAV-GFP-injected mice (n = 10/group). However, AAV-HDAC1-GFP-injected mice displayed significantly reduced fear extinction upon E2 and E3 exposure indicating facilitated fear extinction (**p < 0.01, ***p < 0.0001 vs AAV-GFP). Hippocampus was isolated and prepared for molecular analysis 1 h after E3 exposure (n = 10/group). C, ChIP analysis of the c-Fos promoter in AAV-HDAC1-GFP and AAV-GFP-injected mice. *p < 0.05 vs AAV-GFP). D, qPCR shows decreased hippocampal c-Fos levels in the AAV-HDAC1-GFP group. *p < 0.05 vs AAV-GFP. Error bars indicate SEM.
Figure 8.
Inhibition of HDAC1 activity during fear extinction increases H3K9ac and c-Fos expression. A, Mice that were injected intrahippocampally with MS-275 after each extinction trial exhibited impaired fear extinction when compared with vehicle-treated mice (n = 5/group; p < 0.01). Hippocampal tissue was prepared for molecular analysis 1 h after exposure to E5. B, ChIP analysis of the c-Fos promoters revealed elevated H3K9 acetylation and reduced H3K9 trimethylation in MS-275-injected mice when compared with the control group (*p < 0.01 vs vehicle). C, qPCR shows increased hippocampal −cFos and levels in MS-275-treated mice (*p < 0.05 vs vehicle). D–F, Hippocampal tissue that was obtained 1 h after E5 from the same mice used in the behavior experiment shown in Figure 4_D_ was used for molecular analysis. HDAC1 siRNA-treated mice (n = 5) display reduced HDAC1 levels (D), increased H3K9 acetylation and decreased H4K9 methylation (E) at the cFos promoter while cFos expression was increased (F) when compared with scrambled RNA-treated mice (n = 4, *p < 0.05). Error bars indicate SEM.
Figure 9.
Fear extinction-dependent recruitment of HDAC1 to the Egr-2 promoter. Fear extinction training (see Fig. 5_A_) was performed in the mice (n = 40) that were used for the molecular analysis in A and B. A, Egr-2 expression was analyzed via qPCR in hippocampal tissue isolated 1 h after exposure to extinction trials. The data are normalized to tissue obtained from a naive control group. B, HDAC1 ChIP was performed from hippocampal tissue 1 h after exposure to E1, E3, and E5. Note that the downregulation of Egr-2 correlates with recruitment of HDAC1 to the Egr-2 promoter. C, We used the same samples described in Figure 5_D_ to analyze Egr-2 expression and HDAC1 recruitment to the Egr-2 promoter in the E1, the E1–3 d, and the E1–E5 group. We would like to reiterate that freezing behavior in the E1–3 d group was significantly higher when compared with the E1–E5 group. Egr-2 expression was measured 1 h after extinction trials. Egr-2 levels were significantly higher in the E1–3 d group when compared with E1–E5 group. (*p < 0.05 vs E1 and E1–3 d). D, HDAC1 ChIP was performed from hippocampal tissue 1 h after exposure to extinction trial in the E1, E1–3 d, and E1–E5 groups. Note that the increased Egr-2 expression in the E1–3 d group correlates with reduced HDAC1 level at the Egr-2 promoter. *p < 0.05 vs E1 and E1–3 d. Error bars indicate SEM.
Similar articles
- Active, phosphorylated fingolimod inhibits histone deacetylases and facilitates fear extinction memory.
Hait NC, Wise LE, Allegood JC, O'Brien M, Avni D, Reeves TM, Knapp PE, Lu J, Luo C, Miles MF, Milstien S, Lichtman AH, Spiegel S. Hait NC, et al. Nat Neurosci. 2014 Jul;17(7):971-80. doi: 10.1038/nn.3728. Epub 2014 May 25. Nat Neurosci. 2014. PMID: 24859201 Free PMC article. - The Class I HDAC inhibitor RGFP963 enhances consolidation of cued fear extinction.
Bowers ME, Xia B, Carreiro S, Ressler KJ. Bowers ME, et al. Learn Mem. 2015 Mar 16;22(4):225-31. doi: 10.1101/lm.036699.114. Print 2015 Apr. Learn Mem. 2015. PMID: 25776040 Free PMC article. - Regulation of HDAC1 and HDAC2 during consolidation and extinction of fear memory.
Siddiqui SA, Singh S, Ugale R, Ranjan V, Kanojia R, Saha S, Tripathy S, Kumar S, Mehrotra S, Modi DR, Prakash A. Siddiqui SA, et al. Brain Res Bull. 2019 Aug;150:86-101. doi: 10.1016/j.brainresbull.2019.05.011. Epub 2019 May 17. Brain Res Bull. 2019. PMID: 31108155 - Histone acetylation rescues contextual fear conditioning in nNOS KO mice and accelerates extinction of cued fear conditioning in wild type mice.
Itzhak Y, Anderson KL, Kelley JB, Petkov M. Itzhak Y, et al. Neurobiol Learn Mem. 2012 May;97(4):409-17. doi: 10.1016/j.nlm.2012.03.005. Epub 2012 Mar 20. Neurobiol Learn Mem. 2012. PMID: 22452925 Free PMC article. - HDAC1 and HDAC2 collectively regulate intestinal stem cell homeostasis.
Zimberlin CD, Lancini C, Sno R, Rosekrans SL, McLean CM, Vlaming H, van den Brink GR, Bots M, Medema JP, Dannenberg JH. Zimberlin CD, et al. FASEB J. 2015 May;29(5):2070-80. doi: 10.1096/fj.14-257931. Epub 2015 Feb 3. FASEB J. 2015. PMID: 25648995
Cited by
- The potential of epigenetics in stress-enhanced fear learning models of PTSD.
Blouin AM, Sillivan SE, Joseph NF, Miller CA. Blouin AM, et al. Learn Mem. 2016 Sep 15;23(10):576-86. doi: 10.1101/lm.040485.115. Print 2016 Oct. Learn Mem. 2016. PMID: 27634148 Free PMC article. Review. - Environmental enrichment: aging and memory.
Patel TR. Patel TR. Yale J Biol Med. 2012 Dec;85(4):491-500. Epub 2012 Dec 13. Yale J Biol Med. 2012. PMID: 23239950 Free PMC article. - A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice.
Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. Chatterjee S, et al. J Neurosci. 2013 Jun 26;33(26):10698-712. doi: 10.1523/JNEUROSCI.5772-12.2013. J Neurosci. 2013. PMID: 23804093 Free PMC article. - Genetic Mapping of Behavioral Traits Using the Collaborative Cross Resource.
Xuan W, Zhang L, Zhang Y, Sun X, Wang J, Li X, Zhang L, Wang X, Morahan G, Qin C. Xuan W, et al. Int J Mol Sci. 2022 Dec 30;24(1):682. doi: 10.3390/ijms24010682. Int J Mol Sci. 2022. PMID: 36614124 Free PMC article. - The Contribution of Epigenetic Inheritance Processes on Age-Related Cognitive Decline and Alzheimer's Disease.
Bellver-Sanchis A, Pallàs M, Griñán-Ferré C. Bellver-Sanchis A, et al. Epigenomes. 2021 Jun 18;5(2):15. doi: 10.3390/epigenomes5020015. Epigenomes. 2021. PMID: 34968302 Free PMC article. Review.
References
- Agis-Balboa RC, Arcos-Diaz D, Wittnam J, Govindarajan N, Blom K, Burkhardt S, Haladyniak U, Agbemenyah HY, Zovoilis A, Salinas-Riester G, Opitz L, Sananbenesi F, Fischer A. A hippocampal insulin-growth factor 2 pathway regulates the extinction of fear memories. EMBO J. 2011;30:4071–4083. - PMC - PubMed
- Ajamian F, Salminen A, Reeben M. Selective regulation of class I and class II histone deacetylases expression by inhibitors of histone deacetylases in cultured mouse neural cells. Neurosci Lett. 2004;365:64–68. - PubMed
- Amann LC, Gandal MJ, Halene TB, Ehrlichman RS, White SL, McCarren HS, Siegel SJ. Mouse behavioral endophenotypes for schizophrenia. Brain Res Bull. 2010;30:147–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous