Investigation of mitochondrial dysfunction by sequential microplate-based respiration measurements from intact and permeabilized neurons - PubMed (original) (raw)
Investigation of mitochondrial dysfunction by sequential microplate-based respiration measurements from intact and permeabilized neurons
Pascaline Clerc et al. PLoS One. 2012.
Abstract
Mitochondrial dysfunction is a component of many neurodegenerative conditions. Measurement of oxygen consumption from intact neurons enables evaluation of mitochondrial bioenergetics under conditions that are more physiologically realistic compared to isolated mitochondria. However, mechanistic analysis of mitochondrial function in cells is complicated by changing energy demands and lack of substrate control. Here we describe a technique for sequentially measuring respiration from intact and saponin-permeabilized cortical neurons on single microplates. This technique allows control of substrates to individual electron transport chain complexes following permeabilization, as well as side-by-side comparisons to intact cells. To illustrate the utility of the technique, we demonstrate that inhibition of respiration by the drug KB-R7943 in intact neurons is relieved by delivery of the complex II substrate succinate, but not by complex I substrates, via acute saponin permeabilization. In contrast, methyl succinate, a putative cell permeable complex II substrate, failed to rescue respiration in intact neurons and was a poor complex II substrate in permeabilized cells. Sequential measurements of intact and permeabilized cell respiration should be particularly useful for evaluating indirect mitochondrial toxicity due to drugs or cellular signaling events which cannot be readily studied using isolated mitochondria.
Conflict of interest statement
Competing Interests: BMP has served as a consult for Seahorse Bioscience. Seahorse Bioscience produces a product, the XF24 analyzer, that is used for development of the approach described in this manuscript. This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
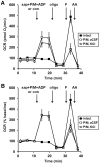
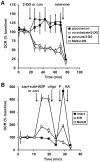
Figure 1. Comparison of saponin-permeabilized neuronal respiration in aCSF vs. KCl-based medium.
(A) Primary rat cortical neurons were control-treated in aCSF (con, filled squares) or permeabilized by saponin (sap, 25 µg/ml) in aCSF (open squares) or KCl-based assay medium (open triangles) after three baseline O2 consumption rate (OCR) measurements (first arrow). Respiration was stimulated by co-injection of ADP (1 mM) in the presence of the complex I substrates pyruvate and malate (P/M, 5 mM each). K2HPO4 was also co-injected with saponin to obtain a final concentration of 4 mM in each assay medium (see Methods) and EGTA (5 mM) was included for cells assayed in aCSF. Oligomycin (oligo, 0.3 µg/ml), FCCP, (F, 2 µM) and antimycin A (AA, 1 µM) were subsequently added as indicated (arrows). Pyruvate (10 mM) was included with FCCP for intact cells (filled squares) here and in subsequent experiments to insure that substrate supply was not rate-limiting for uncoupled respiration. OCRs were measured in triplicate (mean ± SD). (B) OCRs in (A) baseline-normalized to the point prior to sap or con addition.
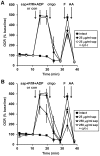
Figure 2. Optimized plasma membrane permeabilization by saponin does not compromise mitochondrial outer membrane integrity.
(A) Primary rat cortical neurons were control-treated (con, filled squares) or permeabilized by saponin (sap, 25 µg/ml) plus EGTA (5 mM) in aCSF medium in the absence (filled circles) or presence (open circles) of purified cytochrome c (100 µM) after three baseline O2 consumption rate (OCR) measurements (first arrow). Pyruvate and malate (P/M, 5 mM each), ADP (1 mM), and excess K2PHO4 (3.6 mM for 4 mM final) were co-injected with saponin to measure complex I-dependent ADP-stimulated respiration. Subsequently, oligomycin (oligo, 0.3 µg/ml), FCCP, (F, 2 µM) and antimycin A (AA, 1 µM) were added as indicated (arrows). Pyruvate (10 mM) was included with FCCP for intact cells (filled squares). (B) Neurons were permeabilized as described in (A) using 250 µg/ml saponin in the absence (filled triangles) or presence (open triangles) of purified cytochrome c (100 µM). Control-treated neurons (filled squares) and neurons permeabilized by 25 µg/ml saponin (filled circles) are duplicated from (A) for comparison. OCRs in (A) and (B) are mean ± SD in quadruplicate, normalized to the third measurement point and expressed as % baseline OCR. All treatments in (A) and (B) were assayed in parallel on an individual 24-well plate.
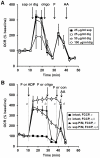
Figure 3. Effect of the permeabilizing agent and time on FCCP-stimulated respiration.
(A) Primary rat cortical neurons were permeabilized by saponin (sap, 25 µg/ml, filled squares) or digitonin (25 µg/ml, open squares, 50 µg/ml, open triangles, 100 µg/ml, open circles) plus EGTA (5 mM) in aCSF medium after three baseline O2 consumption rate (OCR) measurements (first arrow). Pyruvate and malate (P/M, 5 mM each), ADP (1 mM), and excess K2PHO4 (3.6 mM for 4 mM final) were co-injected with saponin to measure complex I-dependent ADP-stimulated respiration. Oligomycin (oligo, 0.3 µg/ml), FCCP, (F, 2 µM) and antimycin A (AA, 1 µM) were added as indicated (arrows). (B) OCRs were measured and at the injection marked a, neurons were control-treated in the absence (filled squares) or presence of FCCP plus pyruvate (2 µM and 10 mM, respectively, open squares) or permeabilized using saponin (triangles). Complex I-linked respiration (P/M) in permeabilized neurons was stimulated by ADP (1 mM, filled triangles) or FCCP (F, 2 µM, open triangles). Intact (open squares) and permeabilized (open triangles) cells treated with FCCP received a second FCCP injection (1 µM) at b to insure respiration was maximally uncoupled, followed by a control injection at c and finally antimycin A (AA, 1 µM) at d. Control-treated intact cells (filled squares) and ADP-treated permeabilized cells (filled triangles) received injections of oligo, FCCP, (F, 2 µM) and AA in ports b, c, and d, respectively, with pyruvate (10 mM) included with FCCP for intact cells. OCRs in (A) and (B) are mean ± SD in quadruplicate, normalized to the third measurement point and expressed as % baseline OCR.
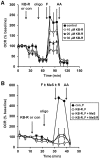
Figure 4. Methyl succinate fails to rescue KB-R7943-inhibited respiration in intact neurons.
(A) Primary rat cortical neurons were control-treated (con, filled squares) or treated with KB-7943 (K-BR, 10 µM, open circles, 20 µM, filled triangles, or 30 µM, open triangles) after three baseline O2 consumption rate (OCR) measurements (first arrow). Subsequently, oligomycin (oligo, 0.3 µg/ml), FCCP plus pyruvate (F, 2 µM and 10 mM, respectively), and antimycin A (AA, 1 µM) were added as indicated (arrows). (B) Neurons were control-treated (con, filled squares) or treated with KB-7943 (30 µM, all other groups) as in (A). Other additions were as (A) except the FCCP+pyruvate injection after KB-R7943 addition also contained methyl succinate (MeS, 10 mM, open triangles), methyl succinate plus rotenone (10 mM/0.5 µM, MeS/R, open circles), or no additional substrate (open squares). OCRs in (A) and (B) are mean ± SD in triplicate, normalized to the third measurement point and expressed as % baseline OCR.
Figure 5. Methyl succinate is a poor substrate for complex II.
(A) Primary rat cortical neurons were incubated in aCSF in the presence of glucose (15 mM, filled squares), no substrate (open circles), pyruvate (10 mM, filled triangles), or methyl succinate (10 mM, open squares). Cells were control-treated (con, filled squares) or treated with 2-deoxyglucose (2-DG, 2 mM, all other groups) after three baseline O2 consumption rate (OCR) measurements (first arrow). Rotenone (0.5 µM) was subsequently added (second arrow) to inhibit complex I. (B) Neurons were control-treated (con, filled squares) or permeabilized by saponin (sap, 25 µg/ml) plus EGTA (5 mM) in aCSF medium after three baseline O2 consumption rate (OCR) measurements (first arrow). Succinate/rotenone (S/R, 5 mM and 0.5 mM respectively) or methyl succinate/rotenone (MeS/R, 5 mM and 0.5 mM respectively) as substrate (sub), ADP (1 mM), and excess K2PHO4 (3.6 mM for 4 mM final) were co-injected with saponin to measure complex II-dependent ADP-stimulated respiration. Subsequently, oligomycin (oligo, 0.3 µg/ml), FCCP, (F, 2 µM) and antimycin A (AA, 1 µM) were added as indicated (arrows). Pyruvate (10 mM) was included with FCCP for intact cells (filled squares) to insure that substrate supply was not rate-limiting for uncoupled respiration. OCRs in (A) and (B) are mean ± SD in triplicate, normalized to the third measurement point and expressed as % baseline OCR.
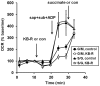
Figure 6. Inhibition of respiration by KB-R7943 is reversed by the complex II substrate succinate.
Primary rat cortical neurons were control-treated (filled symbols) or treated with KB-7943 (K-BR, 30 µM, open symbols) after three baseline O2 consumption rate (OCR) measurements (first arrow). Neurons were then permeabilized by saponin (sap, 25 µg/ml) plus EGTA (5 mM) in aCSF medium containing glutamate/malate (G/M, 3 mM and 1 mM, respectively, circles) or succinate/glutamate (S/G, 3 mM each, triangles) as substrates (sub), ADP (1 mM), and excess K2PHO4 (3.6 mM for 4 mM final) to measure ADP-stimulated respiration (second arrow). Neurons permeabilized in the presence of G/M received a subsequent succinate (5 mM) addition (third arrow, circles) while neurons already exposed to succinate received a control (con) injection (triangles). OCRs are mean ± SD in triplicate, normalized to the third measurement point and expressed as % baseline OCR.
Similar articles
- Mitochondrial respiratory chain complex I dysfunction induced by N-methyl carbamate ex vivo can be alleviated with a cell-permeable succinate prodrug.
Janowska JI, Piel S, Saliba N, Kim CD, Jang DH, Karlsson M, Kilbaugh TJ, Ehinger JK. Janowska JI, et al. Toxicol In Vitro. 2020 Jun;65:104794. doi: 10.1016/j.tiv.2020.104794. Epub 2020 Feb 11. Toxicol In Vitro. 2020. PMID: 32057835 Free PMC article. - Chromium(VI) interaction with plant and animal mitochondrial bioenergetics: a comparative study.
Fernandes MA, Santos MS, Alpoim MC, Madeira VM, Vicente JA. Fernandes MA, et al. J Biochem Mol Toxicol. 2002;16(2):53-63. doi: 10.1002/jbt.10025. J Biochem Mol Toxicol. 2002. PMID: 11979422 - Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I.
El-Mir MY, Nogueira V, Fontaine E, Avéret N, Rigoulet M, Leverve X. El-Mir MY, et al. J Biol Chem. 2000 Jan 7;275(1):223-8. doi: 10.1074/jbc.275.1.223. J Biol Chem. 2000. PMID: 10617608 - Active mitochondrial respiration in cancer: a target for the drug.
Bedi M, Ray M, Ghosh A. Bedi M, et al. Mol Cell Biochem. 2022 Feb;477(2):345-361. doi: 10.1007/s11010-021-04281-4. Epub 2021 Oct 30. Mol Cell Biochem. 2022. PMID: 34716860 Review. - Forces, fluxes, and fuels: tracking mitochondrial metabolism by integrating measurements of membrane potential, respiration, and metabolites.
Jones AE, Sheng L, Acevedo A, Veliova M, Shirihai OS, Stiles L, Divakaruni AS. Jones AE, et al. Am J Physiol Cell Physiol. 2021 Jan 1;320(1):C80-C91. doi: 10.1152/ajpcell.00235.2020. Epub 2020 Nov 4. Am J Physiol Cell Physiol. 2021. PMID: 33147057 Free PMC article. Review.
Cited by
- A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements.
Divakaruni AS, Jastroch M. Divakaruni AS, et al. Nat Metab. 2022 Aug;4(8):978-994. doi: 10.1038/s42255-022-00619-4. Epub 2022 Aug 15. Nat Metab. 2022. PMID: 35971004 Free PMC article. Review. - Analysis of Mitochondrial Function, Structure, and Intracellular Organization In Situ in Cardiomyocytes and Skeletal Muscles.
Kuznetsov AV, Javadov S, Margreiter R, Hagenbuchner J, Ausserlechner MJ. Kuznetsov AV, et al. Int J Mol Sci. 2022 Feb 18;23(4):2252. doi: 10.3390/ijms23042252. Int J Mol Sci. 2022. PMID: 35216368 Free PMC article. Review. - Gut-Derived Metabolite Indole-3-Propionic Acid Modulates Mitochondrial Function in Cardiomyocytes and Alters Cardiac Function.
Gesper M, Nonnast ABH, Kumowski N, Stoehr R, Schuett K, Marx N, Kappel BA. Gesper M, et al. Front Med (Lausanne). 2021 Mar 22;8:648259. doi: 10.3389/fmed.2021.648259. eCollection 2021. Front Med (Lausanne). 2021. PMID: 33829028 Free PMC article. - Mithramycin selectively attenuates DNA-damage-induced neuronal cell death.
Makarevich O, Sabirzhanov B, Aubrecht TG, Glaser EP, Polster BM, Henry RJ, Faden AI, Stoica BA. Makarevich O, et al. Cell Death Dis. 2020 Jul 27;11(7):587. doi: 10.1038/s41419-020-02774-6. Cell Death Dis. 2020. PMID: 32719328 Free PMC article. - A New Pathway Promotes Adaptation of Human Glioblastoma Cells to Glucose Starvation.
Azzalin A, Brambilla F, Arbustini E, Basello K, Speciani A, Mauri P, Bezzi P, Magrassi L. Azzalin A, et al. Cells. 2020 May 18;9(5):1249. doi: 10.3390/cells9051249. Cells. 2020. PMID: 32443613 Free PMC article.
References
- Polster BM, Fiskum G. Mitochondrial mechanisms of neural cell apoptosis. J Neurochem. 2004;90:1281–1289. - PubMed
- Fiskum G, Murphy AN, Beal MF. Mitochondria in neurodegeneration: acute ischemia and chronic neurodegenerative diseases. J Cereb Blood Flow Metab. 1999;19:351–369. - PubMed
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
- Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, et al. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HD016596/HD/NICHD NIH HHS/United States
- R01 NS064978/NS/NINDS NIH HHS/United States
- R01NS064978/NS/NINDS NIH HHS/United States
- P01HD016596/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources