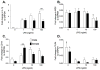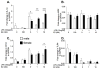Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats - PubMed (original) (raw)
Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats
Lisa C Loram et al. Psychoneuroendocrinology. 2012 Oct.
Abstract
There is a greater prevalence of neuroinflammatory diseases in females than males. Microglia, the major immunocompetent cells of the central nervous system, play a key role in neuroinflammation. We aimed to determine if inherent differences in toll-like receptor 4 mediated pro-inflammatory response in glia could possibly contribute to the skewed female prevalence of neuroinflammatory disorders. In addition, in order to identify if estradiol (E2), the major female sex steroid contributes to a heightened pro-inflammatory response, estradiol was added both in vivo and in vitro. Microglia and astrocytes were isolated from neonatal pups and stimulated with lipopolysaccharide (LPS) in the presence and absence of E2. Hippocampal microglia were isolated from adult male and female rats and stimulated ex vivo with LPS. Male neonatal microglia and astrocytes produced greater IL-1β mRNA than females. However, when co-incubated with varying doses of estradiol (E2), the E2 produced anti-inflammatory effects in the male microglia but a pro-inflammatory effect in female microglia. LPS-induced IL-1β mRNA was attenuated by E2 in female but not male adult hippocampal microglia. However, females supplemented with E2 in vivo produced a potentiated IL-1β mRNA response. TLR4 mRNA was decreased by LPS in both microglia and astrocytes but was not affected by sex or E2. CD14 mRNA was increased by LPS and may be elevated more in females than males in microglia but not astrocytes. Therefore, sexual dimorphic differences do occur in both neonatal and adult microglia though maturity of the microglia at the time of isolation influences the pro-inflammatory response.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
Figure 1
Neonatal male and female microglia isolated from P0/1 pups were incubated with 0, 1, 10 or 100ng/ml LPS for 4 h. IL-1β mRNA (A), MD2 mRNA (B), CD14 mRNA (C) and TLR4 mRNA (D) was measured. There was a significant LPS dose response on IL-1β mRNA and TLR4 mRNA suppression in male but not female microglia. Males produced a greater IL-1β mRNA response than females while females produced a greater CD14 mRNA response at the lowest dose of LPS. Data are expressed as mean ± SEM (n=9–12/gp). ** P<0.01 between groups. $ P<0.05 compared to other doses of LPS.
Figure 2
Neonatal microglia were isolated from male and female P0/1 pups and incubated with vehicle or 10ng/ml LPS and 0, 10 nM or 100 nM 17-β estradiol (E2) for 4 h. IL-1β, MD2, CD14 and TLR4 mRNA were measured. E2 had a significant effect on IL-1β mRNA in both male and female microglia. E2 potentiated the IL-1β mRNA response to LPS in females but attenuated the IL-1β mRNA response in the male microglia. Similar trends were identified in CD14 mRNA expression. There was no significant effect on MD2 mRNA expression, but TLR4 mRNA expression was suppressed by LPS in both sexes. Data are expressed as mean ± SEM (n=9–12/gp).* P<0.05, ** P<0.01, *** P<0.001 between groups on post-hoc comparisons.
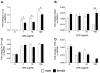
Figure 3
Neonatal male and female astrocytes isolated from P0/1 pups were incubated with 0, 1, 10 and 100ng/ml LPS for 4 h and measured for IL-1β mRNA (A), MD2 mRNA(B), CD14 mRNA (C) and TLR4 mRNA (D). There was a significant LPS dose response on IL-1β mRNA and TLR4 mRNA in both male and female astrocytes. Males produced a greater IL-1β mRNA response than females. There was a significant effect of LPS on CD14 mRNA but no difference between the sexes. There was no significant effect on MD2 except in males at the high dose of LPS. Data are expressed as mean ± SEM (n=6/gp). * P<0.05 between groups.
Figure 4
Neonatal astrocytes isolated from male and female pups that were incubated with vehicle or 10 ng/ml LPS and 0, 10 nM or 100 nM 17-β estradiol (E2) for 4 h. IL-1β, MD2, CD14 and TLR4 mRNA was measured. E2 had no significant effect on IL-1β, MD2, CD14 or TLR4 mRNA in either male and female astrocytes. Data are expressed as mean ± SEM (n=6/gp).** P<0.01, *** P<0.001, between groups.
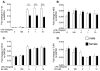
Figure 5
Rapidly isolated hippocampal microglia from adult male and OVX female rats were incubated with 0 or 100 ng/ml LPS and 0, 10 or 100 nM E2 for 3 h. IL-1β, MD2, CD14 and TLR4 mRNA from the cells was measured. There was significantly greater IL-1β and CD14 mRNA in males compared to females. E2 significantly suppressed the IL-1β mRNA response to LPS in female microglia. Females had significantly greater TLR4 mRNA than males. Data are expressed as mean ± SEM (n=6–8 per group). $ P<0.05 for main effect of sex, @ P<0.05 significant effect of LPS/E2 where indicated
Figure 6
Rapidly isolated hippocampal microglia from adult male and OVX ± E2 female rats were incubated with 0, 10 or 100 ng/ml LPS for 3 h. IL-1β, MD2, CD14 and TLR4 mRNA was measured. LPS significantly increased IL-1β and CD14 mRNA in all groups. Supplemented E2 in the OVX females significantly increased IL-1β mRNA compared to all other groups at both doses of LPS. There was a significant LPS dose response on CD14 mRNA, with males and females supplemented with E2 having the greatest CD14 mRNA expression. Females and males with E2 had significantly greater TLR4 mRNA in response to LPS compared to those not supplemented with E2. Data are expressed as mean ± SEM (n=6–10 per group). *** P<0.001 compared to all other groups at the same dose of LPS.
Similar articles
- The sex differences of the behavior response to early Life immune stimulation: Microglia and astrocytes involvement.
Berkiks I, Garcia-Segura LM, Nassiri A, Mesfioui A, Ouichou A, Boulbaroud S, Bahbiti Y, Lopez-Rodriguez AB, Hasnaoui E, El Hessni A. Berkiks I, et al. Physiol Behav. 2019 Feb 1;199:386-394. doi: 10.1016/j.physbeh.2018.11.037. Epub 2018 Dec 7. Physiol Behav. 2019. PMID: 30529512 - Ammonia Attenuates LPS-Induced Upregulation of Pro-Inflammatory Cytokine mRNA in Co-Cultured Astrocytes and Microglia.
Karababa A, Groos-Sahr K, Albrecht U, Keitel V, Shafigullina A, Görg B, Häussinger D. Karababa A, et al. Neurochem Res. 2017 Mar;42(3):737-749. doi: 10.1007/s11064-016-2060-4. Epub 2016 Sep 21. Neurochem Res. 2017. PMID: 27655254 - Modulatory effect of IL-1 inhibition following lipopolysaccharide-induced neuroinflammation in neonatal microglia and astrocytes.
Pierre WC, Londono I, Quiniou C, Chemtob S, Lodygensky GA. Pierre WC, et al. Int J Dev Neurosci. 2022 May;82(3):243-260. doi: 10.1002/jdn.10179. Epub 2022 Mar 27. Int J Dev Neurosci. 2022. PMID: 35315121 - Various roles of astrocytes during recovery from repeated exposure to different doses of lipopolysaccharide.
Bian Y, Zhao X, Li M, Zeng S, Zhao B. Bian Y, et al. Behav Brain Res. 2013 Sep 15;253:253-61. doi: 10.1016/j.bbr.2013.07.028. Epub 2013 Jul 26. Behav Brain Res. 2013. PMID: 23896049 - Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA₂-IIA expression in astrocytes and microglia.
Sheng W, Zong Y, Mohammad A, Ajit D, Cui J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, Hong JS, Weisman GA, Sun GY. Sheng W, et al. J Neuroinflammation. 2011 Sep 24;8:121. doi: 10.1186/1742-2094-8-121. J Neuroinflammation. 2011. PMID: 21943492 Free PMC article.
Cited by
- Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats.
Pyter LM, Kelly SD, Harrell CS, Neigh GN. Pyter LM, et al. Brain Behav Immun. 2013 May;30:88-94. doi: 10.1016/j.bbi.2013.01.075. Epub 2013 Jan 21. Brain Behav Immun. 2013. PMID: 23348027 Free PMC article. - The Pathological Activation of Microglia Is Modulated by Sexually Dimorphic Pathways.
O'Connor JL, Nissen JC. O'Connor JL, et al. Int J Mol Sci. 2023 Mar 1;24(5):4739. doi: 10.3390/ijms24054739. Int J Mol Sci. 2023. PMID: 36902168 Free PMC article. Review. - Ovariectomy and subsequent treatment with estrogen receptor agonists tune the innate immune system of the hippocampus in middle-aged female rats.
Sárvári M, Kalló I, Hrabovszky E, Solymosi N, Liposits Z. Sárvári M, et al. PLoS One. 2014 Feb 13;9(2):e88540. doi: 10.1371/journal.pone.0088540. eCollection 2014. PLoS One. 2014. PMID: 24551115 Free PMC article. - Sex Steroids, Adult Neurogenesis, and Inflammation in CNS Homeostasis, Degeneration, and Repair.
Larson TA. Larson TA. Front Endocrinol (Lausanne). 2018 Apr 30;9:205. doi: 10.3389/fendo.2018.00205. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29760681 Free PMC article. Review. - Examination of sex and minocycline treatment on acute morphine-induced analgesia and inflammatory gene expression along the pain pathway in Sprague-Dawley rats.
Posillico CK, Terasaki LS, Bilbo SD, Schwarz JM. Posillico CK, et al. Biol Sex Differ. 2015 Dec 12;6:33. doi: 10.1186/s13293-015-0049-3. eCollection 2015. Biol Sex Differ. 2015. PMID: 26693004 Free PMC article.
References
- Achiron A, Gurevich M. Gender effects in relapsing-remitting multiple sclerosis: correlation between clinical variables and gene expression molecular pathways. J Neurol Sci. 2009;286:47–53. - PubMed
- Aloisi F. The role of microglia and astrocytes in CNS immune surveillance and immunopathology. Adv Exp Med Biol. 1999;468:123–133. - PubMed
- Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32:1995–2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA024044/DA/NIDA NIH HHS/United States
- DE107782/DE/NIDCR NIH HHS/United States
- K05 DA024044/DA/NIDA NIH HHS/United States
- DA023132/DA/NIDA NIH HHS/United States
- R01 DE017782/DE/NIDCR NIH HHS/United States
- R01 DE021966/DE/NIDCR NIH HHS/United States
- R01 DA023132/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials