A novel antihepatitis drug, bicyclol, prevents liver carcinogenesis in diethylnitrosamine-initiated and phenobarbital-promoted mice tumor model - PubMed (original) (raw)
A novel antihepatitis drug, bicyclol, prevents liver carcinogenesis in diethylnitrosamine-initiated and phenobarbital-promoted mice tumor model
Hua Sun et al. J Biomed Biotechnol. 2012.
Abstract
Bicyclol, an antihepatitis drug developed by Chinese scientists, has been shown to prevent the malignant transformation induced by 3-methylcholanthrene and 12-O-tetradecanoylphorbol-13-acetate in WB-F344 rat liver epithelial cells. This study provides further evidence on its role as a chemopreventive agent in experimental mice with diethylnitrosamine- (DEN-) initiated and phenobarbital- (PB-) promoted liver carcinoma. Liver tissue and serum were collected. In the two-stage model of hepatocarcinogenesis in mice, oral administration of bicyclol (100, 200 mg/kg) before DEN injection showed significant reduction in the incidence of hepatocellular foci, nodules, or carcinoma. Histopathological examination revealed that there was no hepatocellular carcinoma (HCC) and hepatoma formation in the mice pretreated with bicyclol (200 mg/kg) at week 20, while the mice treated with DEN/PB developed 33.3% HCC and 55.6% hepatoma. Furthermore, the serum levels of alanine aminotransferase (ALT), alkaline phosphatase (ALP), and α-fetal protein (AFP) in serum significantly increased in the DEN/PB model group in comparison with the control group. Pretreatment with bicyclol showed a marked reduction in the above condition. Bicyclol also decreased the expression of AFP and proliferating cell nuclear antigen level in the liver tissue and attenuated the decrease in body weight. In this study, we also found that 10 weeks after stopping the administration of PB and drugs, the control and bicyclol-treated (200 mg/kg) animals showed no HCC and hepatoma formation at the time of termination whereas DEN/PB-induced mice developed 100% hepatoma and 50% HCC. These results further indicate that bicyclol has the chemopreventive potential for liver carcinogenesis induced by carcinogens.
Figures
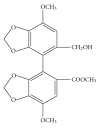
Figure 1
The chemical structure of bicyclol.
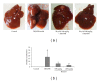
Figure 2
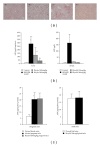
Gross liver tumor and nodule phenotypes in DEN/PB-induced ICR mice and the preventive effect of bicyclol at week 20. (a) Photographs of representative livers. (b) Determination of liver tumor and number of nodules per liver greater than approximately 1 mm in diameter. Average values with standard deviations are shown. n = 8–10. ##P < 0.01 compared with the control group; *P < 0.01, **P < 0.01 compared with the DEN/PB model group.
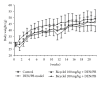
Figure 3
Body weight profile in the control and experimental groups. Average values with standard deviations are shown. n = 14-15.
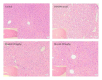
Figure 4
Histological study of the liver tissue obtained from the control and experimental groups at week 20. H&E-stained liver sections were photographed at 100x magnification. Insets in the bottom-left corners are images at 20x magnification of the liver tissue from which the 100x photographs were taken. Control group animals revealed normal architecture. DEN/PB-induced animals showed loss of architecture and presence of cancer cells. Animals pretreated with bicyclol (100, 200 mg/kg) showed fewer neoplastic cells and near-normal architecture.
Figure 5
Expression and quantification of _α_-fetal protein (AFP). (a) AFP-immunoreactivity assay in the control and experimental groups at week 20 using immunohistochemistry assay. (A) control; (B) DEN/PB model; (C) treatment with bicyclol (100 mg/kg) before DEN injection; (D) treatment with bicyclol (200 mg/kg) before DEN injection. (b) Quantification of serum and tissue AFP levels in the control and experimental groups at week 20 using an enzyme immunoassay kit. Average values with standard deviations are shown. n = 8–10. #P < 0.05 compared with the control group; *P < 0.05, **P < 0.01 compared with the DEN/PB model group. (c) Quantification of liver AFP levels in pregnant mice and fetal mice using an enzyme immunoassay kit. Average values with standard deviations are shown. n = 4. #P < 0.05 compared with the control group.
Figure 6
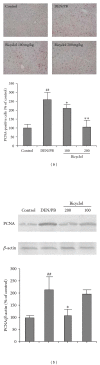
Expression of PCNA in the control and experimental groups at week 20. (a) Representative microscopic pictures of PCNA-stained liver sections by immunohistochemical analysis (100×) and quantification of the immunostained section of PCNA. ##P < 0.01 compared with the control group; *P < 0.05, **P < 0.01 compared with the DEN/PB model group. (b) Western blot assay of PCNA and the quantitative results obtained by measuring the optical density of each band and expressed as the ratio of each targeted protein and beta-actin. Average values of 3 separate experiments along with the standard deviations are shown. ##P < 0.01 compared with the control group; *P < 0.05 compared with the DEN/PB model group.
Similar articles
- Effect of protocatechuic acid-layered double hydroxide nanoparticles on diethylnitrosamine/phenobarbital-induced hepatocellular carcinoma in mice.
Gani SA, Muhammad SA, Kura AU, Barahuie F, Hussein MZ, Fakurazi S. Gani SA, et al. PLoS One. 2019 May 29;14(5):e0217009. doi: 10.1371/journal.pone.0217009. eCollection 2019. PLoS One. 2019. PMID: 31141523 Free PMC article. - Chemoprevention of bicyclol against hepatic preneoplastic lesions.
Zhu B, Liu GT, Wu RS, Strada SJ. Zhu B, et al. Cancer Biol Ther. 2006 Dec;5(12):1665-73. doi: 10.4161/cbt.5.12.3377. Epub 2006 Dec 7. Cancer Biol Ther. 2006. PMID: 17106253 - Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot.
Kapadia GJ, Azuine MA, Sridhar R, Okuda Y, Tsuruta A, Ichiishi E, Mukainake T, Takasaki M, Konoshima T, Nishino H, Tokuda H. Kapadia GJ, et al. Pharmacol Res. 2003 Feb;47(2):141-8. doi: 10.1016/s1043-6618(02)00285-2. Pharmacol Res. 2003. PMID: 12543062
Cited by
- Comprehensive review of dibenzocyclooctadiene lignans from the Schisandra genus: anticancer potential, mechanistic insights and future prospects in oncology.
Jafernik K, Motyka S, Calina D, Sharifi-Rad J, Szopa A. Jafernik K, et al. Chin Med. 2024 Jan 24;19(1):17. doi: 10.1186/s13020-024-00879-0. Chin Med. 2024. PMID: 38267965 Free PMC article. Review. - The Traditional Medicine and Modern Medicine from Natural Products.
Yuan H, Ma Q, Ye L, Piao G. Yuan H, et al. Molecules. 2016 Apr 29;21(5):559. doi: 10.3390/molecules21050559. Molecules. 2016. PMID: 27136524 Free PMC article. Review. - Role of Bone Marrow Mesenchymal Stem Cells in the Treatment of CCL4 Induced Liver Fibrosis in Albino Rats: A Histological and Immunohistochemical Study.
Ahmed SK, Mohammed SA, Khalaf G, Fikry H. Ahmed SK, et al. Int J Stem Cells. 2014 Nov;7(2):87-97. doi: 10.15283/ijsc.2014.7.2.87. Int J Stem Cells. 2014. PMID: 25473446 Free PMC article. - Combined Use of Bicyclol and Berberine Alleviates Mouse Nonalcoholic Fatty Liver Disease.
Li H, Liu NN, Li JR, Dong B, Wang MX, Tan JL, Wang XK, Jiang J, Lei L, Li HY, Sun H, Jiang JD, Peng ZG. Li H, et al. Front Pharmacol. 2022 Feb 16;13:843872. doi: 10.3389/fphar.2022.843872. eCollection 2022. Front Pharmacol. 2022. PMID: 35250593 Free PMC article. - Metformin Improves Biochemical and Pathophysiological Changes in Hepatocellular Carcinoma with Pre-Existed Diabetes Mellitus Rats.
Mobasher MA, Germoush MO, Galal El-Tantawi H, Samy El-Said K. Mobasher MA, et al. Pathogens. 2021 Jan 11;10(1):59. doi: 10.3390/pathogens10010059. Pathogens. 2021. PMID: 33440701 Free PMC article.
References
- Feitelson MA, Pan J, Lian Z. Early molecular and genetic determinants of primary liver malignancy. Surgical Clinics of North America. 2004;84(2):339–354. - PubMed
- Yerian LM, Anders RA, Tretiakova M, Hart J. Caveolin and thrombospondin expression during hepatocellular carcinogenesis. American Journal of Surgical Pathology. 2004;28(3):357–364. - PubMed
- Birrer RB, Birrer D, Klavins JV. Review: hepatocellular carcinoma and hepatitis virus. Annals of Clinical and Laboratory Science. 2003;33(1):39–54. - PubMed
- Brown RS, Gaglio PJ. Scope of worldwide hepatitis C problem. Liver Transplantation. 2003;9(11):S10–S13. - PubMed
- Glinghammar B, Shogsberg J, Hamsten A, Ehrenborg E. PPARδ activation induces COX-2 gene expression and cell proliferation in human hepatocellular carcinoma cells. Biochemical and Biophysical Research Communications. 2003;308:361–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources