Membrane dynamics correlate with formation of signaling clusters during cell spreading - PubMed (original) (raw)
Membrane dynamics correlate with formation of signaling clusters during cell spreading
King Lam Hui et al. Biophys J. 2012.
Abstract
The morphology and duration of contacts between cells and adhesive surfaces play a key role in several biological processes, such as cell migration, cell differentiation, and the immune response. The interaction of receptors on the cell membrane with ligands on the adhesive surface leads to triggering of signaling pathways, which allow cytoskeletal rearrangement, and large-scale deformation of the cell membrane, which allows the cell to spread over the substrate. Despite numerous studies of cell spreading, the nanometer-scale dynamics of the membrane during formation of contacts, spreading, and initiation of signaling are not well understood. Using interference reflection microscopy, we study the kinetics of cell spreading at the micron scale, as well as the topography and fluctuations of the membrane at the nanometer scale during spreading of Jurkat T cells on antibody-coated substrates. We observed two modes of spreading, which were characterized by dramatic differences in membrane dynamics and topography. Formation of signaling clusters was closely related to the movement and morphology of the membrane in contact with the activating surface. Our results suggest that cell membrane morphology may be a critical constraint on signaling at the cell-substrate interface.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
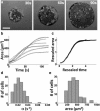
Figure 1
Spreading of Jurkat T lymphocytes on antibody-coated substrates. (a) Time-lapse IRM images showing the increasing contact zone as the cell spreads out. Scale bar, 5 _μ_m. (b) Contact area as a function of time for six representative cells. The smooth lines are fits to a tanh function, A(t) ∼ _A_0 tanh(αt). (c) Rescaled graphs showing that the spreading of all cells can be described by a common spreading function. (d) Histogram of the spreading rate, α (n = 88). (e) Histogram of the final spread area _A_0.
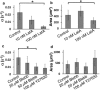
Figure 2
Cells with compromised actin polymerization do not spread efficiently. (a) Spreading rate (α) decreases as the concentration of Lat-A increases (p < 0.01, _t_-test). (_b_) The final spread area is unaffected for small concentrations of Lat-A, but is much smaller for higher concentrations (_p_ < 0.05, _t_-test). (_c_) Spreading rate is diminished upon inhibition of the activity of NMMII or Rho kinase (ROCK). (_d_) Final spread area is not affected by inhibition of NMMII or ROCK. The number of cells analyzed was >18 in all conditions.
Figure 3
Spreading in the presence of serum is qualitatively different. (a) Time-lapse IRM images of a T cell spreading on antibody-coated glass substrate in the presence of serum. Scale bar, 5 _μ_m. (b) Contact area of the cell as a function of time. Each graph corresponds to a different cell. The solid gray lines show a fit to a hyperbolic tangent function. (c) Comparison of spreading rate, α, and final spread area, _A_0, for serum and serum-free cases shows that these parameters are very similar in the two conditions. (d) Kymographs of four representative sections in serum. (e) Kymographs of two representative sections in serum-free conditions (scale bars, 3 _μ_m, 30 s).
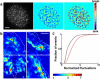
Figure 4
Cell-contact topography is different in the two spreading modes. (a) Denoised IRM images for cells in serum-free media, and (b) in serum containing media. (c) Heat map showing intensity fluctuations relative to shot noise for cells in serum-free media. (d) Heat map showing intensity fluctuations relative to shot noise for cells in serum containing media. (e) Population histograms of relative fluctuation amplitudes, u⌢, in serum-free and (f) in serum. Note: The color bars in both cases represent fluctuation amplitudes relative to shot noise. Scale bars, 5 _μ_m; n = 15 for each condition.
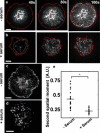
Figure 5
Membrane topography and dynamics of contact are correlated with signaling. (a) Left: TIRF image showing ZAP70 clusters; middle: denoised IRM intensity map showing relative membrane height; right: contours in red show ZAP70 clusters superimposed on the height map. Scale bar, 5 _μ_m. (b) ZAP70 clusters (contours in black) superimposed on regions of low fluctuation, shown in four typical zoomed-in sections of the contact zone from four different cells in serum-supplemented medium. Scale bar, 2 _μ_m. (c) Cumulative histogram of the normalized height fluctuations (black, serum-free; red, serum-supplelmented) for pixels belonging to ZAP70 clusters (10 cells for each condition).
Figure 6
Dynamics and spatial distribution of ZAP70 clusters is distinct in the two modes of spreading. (a) Time-lapse TIRF image of T cells expressing YFP-ZAP70 spreading on an antibody-coated glass substrate in serum-free media. The cyan (inner) line represents the boundary of the clustered zone at any given time point. The red (outer) line denotes the final boundary of the fully spread cell. (b) Time-lapse TIRF image of T cells spreading in serum. To facilitate comparison, the three time-lapse images shown are at 40 s, 80 s, and 160 s (corresponding to rescaled times of 1, 2, and 4). (c) TIRF image of a typical YFP-ZAP70 cell in serum-free media. (d) TIRF image of a typical YFP-ZAP70 cell in serum containing media. Scale bars, 5 _μ_m. (e) Intensity-weighted second spatial moment of fluorescent clusters show that ZAP70 clusters are more peripheral in serum-free conditions but uniformly distributed in serum (p < 0.001, Wilcoxon rank test).
Similar articles
- Adhesion-dependent modulation of actin dynamics in Jurkat T cells.
Lam Hui K, Kwak SI, Upadhyaya A. Lam Hui K, et al. Cytoskeleton (Hoboken). 2014 Feb;71(2):119-35. doi: 10.1002/cm.21156. Epub 2013 Dec 31. Cytoskeleton (Hoboken). 2014. PMID: 24382832 - Cell adhesion and spreading on fluid membranes through microtubules-dependent mechanotransduction.
Mikhajlov O, Adar RM, Tătulea-Codrean M, Macé AS, Manzi J, Tabarin F, Battistella A, di Federico F, Joanny JF, Tran van Nhieu G, Bassereau P. Mikhajlov O, et al. Nat Commun. 2025 Jan 31;16(1):1201. doi: 10.1038/s41467-025-56343-6. Nat Commun. 2025. PMID: 39885125 Free PMC article. - The Actomyosin Cytoskeleton Drives Micron-Scale Membrane Remodeling In Vivo Via the Generation of Mechanical Forces to Balance Membrane Tension Gradients.
Ebrahim S, Liu J, Weigert R. Ebrahim S, et al. Bioessays. 2018 Sep;40(9):e1800032. doi: 10.1002/bies.201800032. Epub 2018 Aug 6. Bioessays. 2018. PMID: 30080263 Free PMC article. Review. - Signaling network triggers and membrane physical properties control the actin cytoskeleton-driven isotropic phase of cell spreading.
Rangamani P, Fardin MA, Xiong Y, Lipshtat A, Rossier O, Sheetz MP, Iyengar R. Rangamani P, et al. Biophys J. 2011 Feb 16;100(4):845-57. doi: 10.1016/j.bpj.2010.12.3732. Biophys J. 2011. PMID: 21320428 Free PMC article. - The Role of Membrane Curvature in Nanoscale Topography-Induced Intracellular Signaling.
Lou HY, Zhao W, Zeng Y, Cui B. Lou HY, et al. Acc Chem Res. 2018 May 15;51(5):1046-1053. doi: 10.1021/acs.accounts.7b00594. Epub 2018 Apr 12. Acc Chem Res. 2018. PMID: 29648779 Free PMC article. Review.
Cited by
- WASp Is Crucial for the Unique Architecture of the Immunological Synapse in Germinal Center B-Cells.
Li Y, Bhanja A, Upadhyaya A, Zhao X, Song W. Li Y, et al. Front Cell Dev Biol. 2021 Jun 14;9:646077. doi: 10.3389/fcell.2021.646077. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34195186 Free PMC article. - Ligand-mediated friction determines morphodynamics of spreading T cells.
Dillard P, Varma R, Sengupta K, Limozin L. Dillard P, et al. Biophys J. 2014 Dec 2;107(11):2629-38. doi: 10.1016/j.bpj.2014.10.044. Epub 2014 Dec 2. Biophys J. 2014. PMID: 25468342 Free PMC article. - Cytoskeletal forces during signaling activation in Jurkat T-cells.
Hui KL, Balagopalan L, Samelson LE, Upadhyaya A. Hui KL, et al. Mol Biol Cell. 2015 Feb 15;26(4):685-95. doi: 10.1091/mbc.E14-03-0830. Epub 2014 Dec 17. Mol Biol Cell. 2015. PMID: 25518938 Free PMC article. - Mechanosensing in the immune response.
Upadhyaya A. Upadhyaya A. Semin Cell Dev Biol. 2017 Nov;71:137-145. doi: 10.1016/j.semcdb.2017.08.031. Epub 2017 Aug 19. Semin Cell Dev Biol. 2017. PMID: 28830744 Free PMC article. Review. - Correlative three-dimensional super-resolution and block-face electron microscopy of whole vitreously frozen cells.
Hoffman DP, Shtengel G, Xu CS, Campbell KR, Freeman M, Wang L, Milkie DE, Pasolli HA, Iyer N, Bogovic JA, Stabley DR, Shirinifard A, Pang S, Peale D, Schaefer K, Pomp W, Chang CL, Lippincott-Schwartz J, Kirchhausen T, Solecki DJ, Betzig E, Hess HF. Hoffman DP, et al. Science. 2020 Jan 17;367(6475):eaaz5357. doi: 10.1126/science.aaz5357. Science. 2020. PMID: 31949053 Free PMC article.
References
- Dustin M.L., Cooper J.A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 2000;1:23–29. - PubMed
- Chamaraux F., Fache S., Fourcade B. Kinetics of cell spreading. Phys. Rev. Lett. 2005;94:158102. - PubMed
- Cuvelier D., Théry M., Mahadevan L. The universal dynamics of cell spreading. Curr. Biol. 2007;17:694–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources