How does the macula protect itself from oxidative stress? - PubMed (original) (raw)
Review
How does the macula protect itself from oxidative stress?
James T Handa. Mol Aspects Med. 2012 Aug.
Abstract
Oxidative stress has been hypothesized to contribute to the development of age-related macular degeneration (AMD), the most common cause of blindness in the United States. At present, there is no treatment for early disease. Reactive oxygen species (ROS) play a physiological role in the retinal pigment epithelium (RPE), a key cell type in this disease, but with excessive ROS, oxidative damage or excessive innate immune system activation can result. The RPE has developed a robust antioxidant system driven by the transcription factor Nrf2. Impaired Nrf2 signaling can lead to oxidative damage or activate the innate immune response, both of which can lead to RPE apoptosis, a defining change in AMD. Several mouse models simulating environmental stressors or targeting specific antioxidant enzymes such as superoxide dismutase or Nrf2, have simulated some of the features of AMD. While ROS are short-lived, oxidatively damaged molecules termed oxidation specific epitopes (OSEs), can be long-lived and a source of chronic stress that activates the innate immune system through pattern recognition receptors (PRRs). The macula accumulates a number of OSEs including carboxyethylpyrrole, malondialdehyde, 4-hydroxynonenal, and advanced glycation endproducts, as well as their respective neutralizing PRRs. Excessive accumulation of OSEs results in pathologic immune activation. For example, mice immunized with the carboxyethylpyrrole develop cardinal features of AMD. Regulating ROS in the RPE by modulating antioxidant systems or neutralizing OSEs through an appropriate innate immune response are potential modalities to treat or prevent early AMD.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures
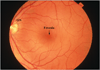
Figure 1
a) Color fundus photograph of a normal left macula. ON, optic nerve. b) Photomicrograph of a normal macula. Note the multilayer ganglion cell layer (GCL). NFL, nerve fiber layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PR, photoreceptor layer; RPE, retinal pigment epithelium; BrM, Bruch’s membrane. Bar=50µm.
Figure 1
a) Color fundus photograph of a normal left macula. ON, optic nerve. b) Photomicrograph of a normal macula. Note the multilayer ganglion cell layer (GCL). NFL, nerve fiber layer; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; PR, photoreceptor layer; RPE, retinal pigment epithelium; BrM, Bruch’s membrane. Bar=50µm.
Figure 2
Fundus photograph of a left macula with many large drusen. The area of greatest involvement is outlined by the arrows.
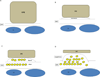
Figure 3
Schematic diagram of degenerative changes to the RPE and Bruch’s membrane during the progression of AMD. A) Normal cuboidal morphology of the RPE. Bruch’s membrane (BrM) is normal thickness. CC, choriocapillaris. B) Flattening of the RPE with early basal laminar deposit (BlamD). C) Further flattening of the RPE with development of more advanced basal laminar deposits and development of basal linear deposits (BlinD). Heterogeneous debris (Yellow deposits) accumulates in both BlamD and BlinD. D) Atrophic RPE overlying large druse.
Figure 4
Photomicrograph of drusen, highlighted by the arrows. Bar=50µm.
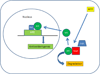
Figure 5
Schematic diagram of Nrf2 signaling. ARE, antioxidant response element; Cul3, Cullin3-dependent E3 ubiquitin ligase complex; Keap1, Kelch-like ECH-associated protein 1; Nrf2, Nuclear factor erythroid-2 related factor 2; Maf, musculoaponeurotic fibrosarcoma protein; ROS, reactive oxygen species.
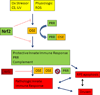
Figure 6
Proposed scheme of oxidative stress and its antioxidant systems including the Nrf2 signaling system and the innate immune system’s activation by oxidation specific epitopes (OSEs). CS, cigarette smoking; PRR, pattern recognition receptor; ROS, reactive oxygen species; UV, photo-oxidative stress.
Similar articles
- Oxidative stress damage circumscribed to the central temporal retinal pigment epithelium in early experimental non-exudative age-related macular degeneration.
Dieguez HH, Romeo HE, Alaimo A, González Fleitas MF, Aranda ML, Rosenstein RE, Dorfman D. Dieguez HH, et al. Free Radic Biol Med. 2019 Feb 1;131:72-80. doi: 10.1016/j.freeradbiomed.2018.11.035. Epub 2018 Nov 28. Free Radic Biol Med. 2019. PMID: 30502459 - Nrf2 signaling is impaired in the aging RPE given an oxidative insult.
Sachdeva MM, Cano M, Handa JT. Sachdeva MM, et al. Exp Eye Res. 2014 Feb;119:111-4. doi: 10.1016/j.exer.2013.10.024. Epub 2013 Nov 8. Exp Eye Res. 2014. PMID: 24216314 Free PMC article. Review. - MiR-125b attenuates retinal pigment epithelium oxidative damage via targeting Nrf2/HIF-1α signal pathway.
Liu JX, Ma DY, Zhi XY, Wang MW, Zhao JY, Qin Y. Liu JX, et al. Exp Cell Res. 2022 Jan 1;410(1):112955. doi: 10.1016/j.yexcr.2021.112955. Epub 2021 Dec 5. Exp Cell Res. 2022. PMID: 34875217 - ID2 protects retinal pigment epithelium cells from oxidative damage through p-ERK1/2/ID2/NRF2.
Fan Y, Huang Z, Long C, Ning J, Zhang H, Kuang X, Zhang Q, Shen H. Fan Y, et al. Arch Biochem Biophys. 2018 Jul 15;650:1-13. doi: 10.1016/j.abb.2018.05.008. Epub 2018 May 16. Arch Biochem Biophys. 2018. PMID: 29753724 - The impact of oxidative stress and inflammation on RPE degeneration in non-neovascular AMD.
Datta S, Cano M, Ebrahimi K, Wang L, Handa JT. Datta S, et al. Prog Retin Eye Res. 2017 Sep;60:201-218. doi: 10.1016/j.preteyeres.2017.03.002. Epub 2017 Mar 20. Prog Retin Eye Res. 2017. PMID: 28336424 Free PMC article. Review.
Cited by
- Immunogenetic and Environmental Factors in Age-Related Macular Disease.
Brodzka S, Baszyński J, Rektor K, Hołderna-Bona K, Stanek E, Kurhaluk N, Tkaczenko H, Malukiewicz G, Woźniak A, Kamiński P. Brodzka S, et al. Int J Mol Sci. 2024 Jun 14;25(12):6567. doi: 10.3390/ijms25126567. Int J Mol Sci. 2024. PMID: 38928273 Free PMC article. Review. - The marine n-3 PUFA DHA evokes cytoprotection against oxidative stress and protein misfolding by inducing autophagy and NFE2L2 in human retinal pigment epithelial cells.
Johansson I, Monsen VT, Pettersen K, Mildenberger J, Misund K, Kaarniranta K, Schønberg S, Bjørkøy G. Johansson I, et al. Autophagy. 2015;11(9):1636-51. doi: 10.1080/15548627.2015.1061170. Autophagy. 2015. PMID: 26237736 Free PMC article. - Adenosine Triphosphate (ATP) and Protein Aggregation in Age-Related Vision-Threatening Ocular Diseases.
Greiner JV, Glonek T. Greiner JV, et al. Metabolites. 2023 Oct 20;13(10):1100. doi: 10.3390/metabo13101100. Metabolites. 2023. PMID: 37887425 Free PMC article. - Common cell biologic and biochemical changes in aging and age-related diseases of the eye: toward new therapeutic approaches to age-related ocular diseases.
Whitcomb EA, Shang F, Taylor A. Whitcomb EA, et al. Invest Ophthalmol Vis Sci. 2013 Dec 13;54(14):ORSF31-6. doi: 10.1167/iovs.13-12808. Invest Ophthalmol Vis Sci. 2013. PMID: 24335065 Free PMC article. Review. No abstract available. - Iron promotes oxidative cell death caused by bisretinoids of retina.
Ueda K, Kim HJ, Zhao J, Song Y, Dunaief JL, Sparrow JR. Ueda K, et al. Proc Natl Acad Sci U S A. 2018 May 8;115(19):4963-4968. doi: 10.1073/pnas.1722601115. Epub 2018 Apr 23. Proc Natl Acad Sci U S A. 2018. PMID: 29686088 Free PMC article.
References
- Abdelsalam A, Del Priore L, Zarbin MA. Drusen in age-related macular degeneration: pathogenesis, natural course, and laser photocoagulation-induced regression. Surv Ophthalmol. 1999;44(1):1–29. - PubMed
- Adams J, Kelso R, Cooley L. The kelch repeat superfamily of proteins: propellers of cell function. Trends Cell Biol. 2000;10(1):17–24. - PubMed
- Age-related Eye Disease Study Research Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. - PMC - PubMed
- Amarilyo G, Verbovetski I, Atallah M, Grau A, Wiser G, Gil O, Ben-Neriah Y, Mevorach D. iC3b-opsonized apoptotic cells mediate a distinct anti-inflammatory response and transcriptional NF-kappaB-dependent blockade. Eur J Immunol. 2010;40(3):699–709. - PubMed
- Anderson DH, Mullins RF, Hageman GS, Johnson LV. A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol. 2002;134(3):411–431. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources