Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins - PubMed (original) (raw)
Cancer cell adhesion and metastasis: selectins, integrins, and the inhibitory potential of heparins
Gerd Bendas et al. Int J Cell Biol. 2012.
Abstract
Cell adhesion molecules play a significant role in cancer progression and metastasis. Cell-cell interactions of cancer cells with endothelium determine the metastatic spread. In addition, direct tumor cell interactions with platelets, leukocytes, and soluble components significantly contribute to cancer cell adhesion, extravasation, and the establishment of metastatic lesions. Clinical evidence indicates that heparin, commonly used for treatment of thromboembolic events in cancer patients, is beneficial for their survival. Preclinical studies confirm that heparin possesses antimetastatic activities that lead to attenuation of metastasis in various animal models. Heparin contains several biological activities that may affect several steps in metastatic cascade. Here we focus on the role of cellular adhesion receptors in the metastatic cascade and discuss evidence for heparin as an inhibitor of cell adhesion. While P- and L-selectin facilitation of cellular contacts during hematogenous metastasis is being accepted as a potential target of heparin, here we propose that heparin may also interfere with integrin activity and thereby affect cancer progression. This review summarizes recent findings about potential mechanisms of tumor cell interactions in the vasculature and antimetastatic activities of heparin.
Figures
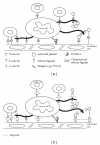
Figure 1
Selectins and integrins contribute to metastatic spread. (a) Schematic presentation of selectin- and integrin-mediated cancer cell interactions with several blood constituents (e.g., platelets, leukocytes, and endothelial cells) during hematogenous metastasis. (b) Heparin application in mouse models blocks both P- and L-selectin-mediated; and VLA4-mediated interactions of cancer cells within blood circulation thereby affecting metastasis.
Similar articles
- Heparanase in Cancer Metastasis - Heparin as a Potential Inhibitor of Cell Adhesion Molecules.
Bendas G, Borsig L. Bendas G, et al. Adv Exp Med Biol. 2020;1221:309-329. doi: 10.1007/978-3-030-34521-1_11. Adv Exp Med Biol. 2020. PMID: 32274715 Review. - Antimetastatic activities of modified heparins: selectin inhibition by heparin attenuates metastasis.
Borsig L. Borsig L. Semin Thromb Hemost. 2007 Jul;33(5):540-6. doi: 10.1055/s-2007-982086. Semin Thromb Hemost. 2007. PMID: 17629852 Review. - Selectins promote tumor metastasis.
Läubli H, Borsig L. Läubli H, et al. Semin Cancer Biol. 2010 Jun;20(3):169-77. doi: 10.1016/j.semcancer.2010.04.005. Epub 2010 May 7. Semin Cancer Biol. 2010. PMID: 20452433 Review. - P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins.
Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, Vlodavsky I, Borsig L. Hostettler N, et al. FASEB J. 2007 Nov;21(13):3562-72. doi: 10.1096/fj.07-8450com. Epub 2007 Jun 8. FASEB J. 2007. PMID: 17557930 - Heparins attenuate cancer metastasis: are selectins the link?
Laubli H, Borsig L. Laubli H, et al. Cancer Invest. 2009 Jun;27(5):474-81. doi: 10.1080/07357900802647136. Cancer Invest. 2009. PMID: 19479484 Review.
Cited by
- Identification of differentially expressed genes and signaling pathways in papillary thyroid cancer: a study based on integrated microarray and bioinformatics analysis.
Sun T, Guan Q, Wang Y, Qian K, Sun W, Ji Q, Wu Y, Guo K, Xiang J. Sun T, et al. Gland Surg. 2021 Feb;10(2):629-644. doi: 10.21037/gs-20-673. Gland Surg. 2021. PMID: 33708546 Free PMC article. - Colorectal Cancer Stem Cells in the Progression to Liver Metastasis.
Gonzalez-Villarreal CA, Quiroz-Reyes AG, Islas JF, Garza-Treviño EN. Gonzalez-Villarreal CA, et al. Front Oncol. 2020 Aug 20;10:1511. doi: 10.3389/fonc.2020.01511. eCollection 2020. Front Oncol. 2020. PMID: 32974184 Free PMC article. Review. - A Combined Activity of Thrombin and P-Selectin Is Essential for Platelet Activation by Pancreatic Cancer Cells.
Haschemi R, Gockel LM, Bendas G, Schlesinger M. Haschemi R, et al. Int J Mol Sci. 2021 Mar 24;22(7):3323. doi: 10.3390/ijms22073323. Int J Mol Sci. 2021. PMID: 33805059 Free PMC article. - CW-EPR studies revealed different motional properties and oligomeric states of the integrin β1a transmembrane domain in detergent micelles or liposomes.
Yu L, Wang W, Ling S, Liu S, Xiao L, Xin Y, Lai C, Xiong Y, Zhang L, Tian C. Yu L, et al. Sci Rep. 2015 Jan 19;5:7848. doi: 10.1038/srep07848. Sci Rep. 2015. PMID: 25597475 Free PMC article. - Molecular characteristics of cancer stem-like cells derived from human breast cancer cells.
Yoo YD, Han DH, Jang JM, Zakrzewska A, Kim SY, Choi CY, Lee YJ, Kwon YT. Yoo YD, et al. Anticancer Res. 2013 Mar;33(3):763-77. Anticancer Res. 2013. PMID: 23482743 Free PMC article.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nature Reviews Cancer. 2002;2(8):563–572. - PubMed
- Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nature Reviews Cancer. 2003;3(6):453–458. - PubMed
- Witz IP. The selectin-selectin ligand axis in tumor progression. Cancer and Metastasis Reviews. 2008;27(1):19–30. - PubMed
- Paschos KA, Canovas D, Bird NC. The role of cell adhesion molecules in the progression of colorectal cancer and the development of liver metastasis. Cellular Signalling. 2009;21(5):665–674. - PubMed
- Läubli H, Borsig L. Selectins promote tumor metastasis. Seminars in Cancer Biology. 2010;20(3):169–177. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources