Impact of PNKP mutations associated with microcephaly, seizures and developmental delay on enzyme activity and DNA strand break repair - PubMed (original) (raw)
Impact of PNKP mutations associated with microcephaly, seizures and developmental delay on enzyme activity and DNA strand break repair
John J Reynolds et al. Nucleic Acids Res. 2012 Aug.
Abstract
Microcephaly with early-onset, intractable seizures and developmental delay (MCSZ) is a hereditary disease caused by mutations in polynucleotide kinase/phosphatase (PNKP), a DNA strand break repair protein with DNA 5'-kinase and DNA 3'-phosphatase activity. To investigate the molecular basis of this disease, we examined the impact of MCSZ mutations on PNKP activity in vitro and in cells. Three of the four mutations currently associated with MCSZ greatly reduce or ablate DNA kinase activity of recombinant PNKP at 30°C (L176F, T424Gfs48X and exon15Δfs4X), but only one of these mutations reduces DNA phosphatase activity under the same conditions (L176F). The fourth mutation (E326K) has little impact on either DNA kinase or DNA phosphatase activity at 30°C, but is less stable than the wild-type enzyme at physiological temperature. Critically, all of the MCSZ mutations identified to date result in ∼ 10-fold reduced cellular levels of PNKP protein, and reduced rates of chromosomal DNA strand break repair. Together, these data suggest that all four known MCSZ mutations reduce the cellular stability and level of PNKP protein, with three mutations likely ablating cellular DNA 5'-kinase activity and all of the mutations greatly reducing cellular DNA 3'-phosphatase activity.
Figures
Figure 1.
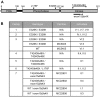
PNKP mutations in MCSZ. (A) Schematic depicting the four PNKP mutations identified in MCSZ, to date. Note that two point mutations (L176F and E326K) are located in the DNA phosphatase domain and two frame-shift mutations (T424Gfs48X and exon15Δfs4X), resulting in premature stop codons and truncated PNKP protein, are located in the DNA kinase domain. FHA denotes the fork-head-associated domain. (B) PNKP genotype of known MCSZ families, unaffected relatives, and associated LCLs used in this study. All individuals and families have been described previously (12).
Figure 2.
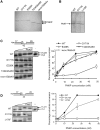
DNA 3′-phosphatase activity of recombinant PNKP harbouring mutations associated with MCSZ. (A and B) Aliquots of the recombinant WT and mutant human PNKP (0.7–1 µg) employed in these experiments was fractionated by SDS–PAGE and visualized by staining with Coomassie brilliant blue. Note that the PNKP proteins depicted in panel A were purified by IMAC/cation exchange and employed in panel C, below, whereas those depicted in panel B were purified by IMAC alone and employed in panel D, below. (C and D) DNA 3′-phosphatase activities of WT and mutant PNKP. 25 nM of the indicated oligonucleotide duplex substrate (top) was incubated without (−) or with 2.5, 10, 25 or 50 nM of WT or the indicated mutant PNKP protein for 60 min at 30°C. Reaction products were fractionated by denaturing PAGE and detected by phosphorimaging. A representative gel is shown on the left, and quantification from four independent experiments (±SEM) on the right. For quantification, the signal intensities of the 17-P substrate and 17-OH product bands were quantified using Aida Image Analyser v.427 and the percentage of 17-OH product calculated. The oligonucleotide duplex contains a 1-bp gap with 3′-phosphate and 5′-phosphate termini. The position of the 3′-phosphate (circled) and 32P-label on the 17-mer substrate-strand are indicated (top).
Figure 3.
DNA 5′-kinase activity of recombinant PNKP harbouring mutations associated with MCSZ. (A and B) DNA 5′-kinase activity of the indicated WT and mutant PNKP. 25 nM of the indicated substrate (top) was incubated without (−) or with 10, 25, 50 or 100 nM of WT or the indicated mutant PNKP for 60 min at 30°C in the presence of [γ-32P] ATP. Reaction products were fractionated by denaturing PAGE and detected by phosphorimaging. Note that in the substrate the 17-mer strand is labelled at the 5′-terminus with 32P to provide an internal loading control. A representative gel is shown on the left and quantification (mean ± SEM) from four independent experiments on the right. For quantification, the signal intensity of the 17-mer and the 25-mer bands were quantified using Aida Image Analyser v.427 and the intensity of the 25-mer normalized by dividing by the intensity of the 17-mer.
Figure 4.
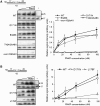
PNKP levels and activity in MCSZ lymphoblastoid cell extracts. (A) Western blot analysis of MCSZ LCL. Samples of the indicated LCLs (2 × 105 cells) were fractionated by SDS–PAGE, transferred to nitrocellulose and immunoblotted for PNKP. Quantification is shown on the right. The signal intensity of PNKP was quantified using Aida Image Analyser v.427 and normalized using the signal intensity of actin. Data are the mean ± SEM of six experiments. Note that, while not included here, PNKP protein levels in MC22601 were similar to its sibling LCL, MC22602. (B) Reduced DNA 3′-phosphatase activity in MCSZ LCL extract. 25 nM single-stranded substrate harbouring a 3′-phosphate terminus (top) was incubated with the indicated LCL extract (2.5 μg total protein) for the indicated time periods at 30°C. Reaction products were fractionated by denaturing PAGE and detected by phosphorimaging. (C) Reduced DNA 3′-phosphatase activity in MCSZ LCL extract. 25 nM duplex substrate harbouring a 1-bp gap and 3′-phosphate terminus (top) was incubated with the indicated LCL extract (1, 2.5, or 7.5 μg total protein) for 60 min at 30°C. Reaction products were analysed as above. Note that DNA 3′-phosphatase activity is indicated by conversion of 17-P to 17-OH and DNA gap filling by conversion of 17-OH to 18-OH. (D) Reduced DNA 5′-kinase activity in MCSZ LCL extract. 25 nM duplex substrate harbouring a nick and 5′-hydroxyl terminus (top) was incubated with the indicated LCL extract (1, 2.5 or 7.5 μg total protein) for 60 min at 30°C. Reaction products were analysed as above. Note that DNA 5′-kinase activity is indicated indirectly in this experiment by appearance of 43-bp DNA ligation product.
Figure 5.
Thermal stability of WT and mutant recombinant histidine-tagged PNKP. (A) Thermal stability of PNKP DNA phosphatase activity. Top left, aliquots of the recombinant WT and mutant human PNKP (0.7 µg) employed in these experiments was fractionated by SDS–PAGE and visualized by staining with Coomassie brilliant blue. Note that the PNKP proteins depicted in this panel were purified by IMAC alone. Middle and bottom, 30 and 60 nM of WT and the indicated mutant PNKP protein were pre-incubated for 2.5 min at the indicated temperatures and then subsequently incubated with 60 nM of the indicated oligonucleotide duplex substrate (top) for 30 min at 30°C. Reaction products were fractionated by denaturing PAGE and detected & by phosphorimaging as described in Figure 2. A representative gel is shown in the middle, and the quantification from four independent experiments (±SEM) at the bottom. (B) Thermal stability of PNKP DNA kinase activity. Top and bottom, 30, 60 and 120 nM of WT and the indicated mutant PNKP protein was pre-incubated for 2.5 min at the indicated temperatures and then subsequently incubated with 60 nM of the indicated oligonucleotide duplex substrate (top) for 30 min at 30°C in the presence of [γ-32P] ATP. Reaction products were fractionated by denaturing PAGE and detected and quantified by phosphorimaging as described in Figure 3. A representative gel is shown at the top and the quantification (mean ± SEM) from four independent experiments at the bottom.
Figure 6.
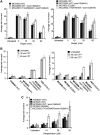
Reduced chromosomal SSBR in MCSZ LCLs. (A) The indicated LCLs were mock-irradiated or γ-irradiated (20 Gy) and incubated for the indicated time periods at 37°C to allow DNA repair. Levels of chromosomal DNA strand breaks were quantified by alkaline comet assays as average tail moments. Data are the mean of 100 cells/time point per experiment and are the average of at least four independent experiments (±SEM). Data sets for the MCSZ LCLs MC22601, MC22602 and MC5403 were significfantly different (two-way ANOVA) to those for the related WT LCLs MC22606 (P = 0.000691), MC22604 (P = 0.00158) and MC5405 (P = 0.00802), respectively. (B) The indicated LCLs were mock-treated or treated with 14 μM CPT for 60 min at 37°C and the level of accumulated chromosomal DNA strand breaks quantified by alkaline comet assays as average tail moments, as described above. Data are the mean of 100 cells/time point and are the average of at three independent experiments (±SEM). DNA sets for the MCSZ LCLs MC22601 and MC22602 were significantly different (two-way ANOVA) to those for the related WT LCLs MC22606 (P = 0.00172) and MC22604 (P = 1.498 E-05), respectively. Note that the LCL line 1635 is from an individual with the Tdp1-mutated disease, SCAN1 and is included as a positive control. (C) The indicated LCLs were mock-treated or treated with 14, 28 or 56 μM CPT for 60 min at 37°C and analysed as above. Only the data set for the MCSZ LCL MC22602 was significantly different from WT (MC22604; P = 3.221 E-06).
Similar articles
- Novel PNKP mutations associated with reduced DNA single-strand break repair and severe microcephaly, seizures, and developmental delay.
Thuresson AC, Brazina J, Akram T, Albrecht J, Dahl N, Soussi Zander C, Caldecott KW. Thuresson AC, et al. Mol Genet Genomic Med. 2024 Jan;12(1):e2295. doi: 10.1002/mgg3.2295. Epub 2023 Nov 2. Mol Genet Genomic Med. 2024. PMID: 37916443 Free PMC article. - Polynucleotide kinase-phosphatase (PNKP) mutations and neurologic disease.
Dumitrache LC, McKinnon PJ. Dumitrache LC, et al. Mech Ageing Dev. 2017 Jan;161(Pt A):121-129. doi: 10.1016/j.mad.2016.04.009. Epub 2016 Apr 26. Mech Ageing Dev. 2017. PMID: 27125728 Free PMC article. Review. - Pathological mutations in PNKP trigger defects in DNA single-strand break repair but not DNA double-strand break repair.
Kalasova I, Hailstone R, Bublitz J, Bogantes J, Hofmann W, Leal A, Hanzlikova H, Caldecott KW. Kalasova I, et al. Nucleic Acids Res. 2020 Jul 9;48(12):6672-6684. doi: 10.1093/nar/gkaa489. Nucleic Acids Res. 2020. PMID: 32504494 Free PMC article. - Mutations of the DNA repair gene PNKP in a patient with microcephaly, seizures, and developmental delay (MCSZ) presenting with a high-grade brain tumor.
Jiang B, Murray C, Cole BL, Glover JNM, Chan GK, Deschenes J, Mani RS, Subedi S, Nerva JD, Wang AC, Lockwood CM, Mefford HC, Leary SES, Ojemann JG, Weinfeld M, Ene CI. Jiang B, et al. Sci Rep. 2022 Mar 30;12(1):5386. doi: 10.1038/s41598-022-09097-w. Sci Rep. 2022. PMID: 35354845 Free PMC article. - Prenatal phenotype of PNKP-related microcephaly, seizures, and developmental delay: A case report and literature review.
Xie JL, Jiang CY, Sun PP, Zhang Y, Sun N, Luan SX. Xie JL, et al. Medicine (Baltimore). 2025 Jan 17;104(3):e41300. doi: 10.1097/MD.0000000000041300. Medicine (Baltimore). 2025. PMID: 39833032 Free PMC article. Review.
Cited by
- Base excision repair in physiology and pathology of the central nervous system.
Bosshard M, Markkanen E, van Loon B. Bosshard M, et al. Int J Mol Sci. 2012 Nov 30;13(12):16172-222. doi: 10.3390/ijms131216172. Int J Mol Sci. 2012. PMID: 23203191 Free PMC article. Review. - Neurological disorders associated with DNA strand-break processing enzymes.
Jiang B, Glover JN, Weinfeld M. Jiang B, et al. Mech Ageing Dev. 2017 Jan;161(Pt A):130-140. doi: 10.1016/j.mad.2016.07.009. Epub 2016 Jul 25. Mech Ageing Dev. 2017. PMID: 27470939 Free PMC article. Review. - DNA repair abnormalities leading to ataxia: shared neurological phenotypes and risk factors.
Gilmore EC. Gilmore EC. Neurogenetics. 2014 Oct;15(4):217-28. doi: 10.1007/s10048-014-0415-z. Epub 2014 Jul 20. Neurogenetics. 2014. PMID: 25038946 Review. - Polynucleotide kinase-phosphatase enables neurogenesis via multiple DNA repair pathways to maintain genome stability.
Shimada M, Dumitrache LC, Russell HR, McKinnon PJ. Shimada M, et al. EMBO J. 2015 Oct 1;34(19):2465-80. doi: 10.15252/embj.201591363. Epub 2015 Aug 19. EMBO J. 2015. PMID: 26290337 Free PMC article. - Genome integrity and disease prevention in the nervous system.
McKinnon PJ. McKinnon PJ. Genes Dev. 2017 Jun 15;31(12):1180-1194. doi: 10.1101/gad.301325.117. Genes Dev. 2017. PMID: 28765160 Free PMC article. Review.
References
- Lindahl T. Instability and decay of the primary structure of DNA. Nature. 1993;362:709–715. - PubMed
- Ward J. In: DNA Damage and Repair Vol2: DNA Repair in Higher Eukaryotes. Ja N, editor. Vol. 2. Totowa, NJ: Human Press Inc; 1998. pp. 65–84.
- Kouzminova EA, Kuzminov A. Fragmentation of replicating chromosomes triggered by uracil in DNA. J. Mol. Biol. 2006;355:20–33. - PubMed
- Beckman KB, Ames BN. Oxidative decay of DNA. J. Biol. Chem. 1997;272:19633–19636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/J006750/1/MRC_/Medical Research Council/United Kingdom
- R01 NS035129/NS/NINDS NIH HHS/United States
- C6563/A10192/CRUK_/Cancer Research UK/United Kingdom
- G0600776/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical