Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells - PubMed (original) (raw)
Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells
Yutaka Maruyama et al. PLoS One. 2012.
Abstract
Recently, we reported that calcium-sensing receptor (CaSR) is a receptor for kokumi substances, which enhance the intensities of salty, sweet and umami tastes. Furthermore, we found that several γ-glutamyl peptides, which are CaSR agonists, are kokumi substances. In this study, we elucidated the receptor cells for kokumi substances, and their physiological properties. For this purpose, we used Calcium Green-1 loaded mouse taste cells in lingual tissue slices and confocal microscopy. Kokumi substances, applied focally around taste pores, induced an increase in the intracellular Ca(2+) concentration ([Ca(2+)](i)) in a subset of taste cells. These responses were inhibited by pretreatment with the CaSR inhibitor, NPS2143. However, the kokumi substance-induced responses did not require extracellular Ca(2+). CaSR-expressing taste cells are a different subset of cells from the T1R3-expressing umami or sweet taste receptor cells. These observations indicate that CaSR-expressing taste cells are the primary detectors of kokumi substances, and that they are an independent population from the influenced basic taste receptor cells, at least in the case of sweet and umami.
Conflict of interest statement
Competing Interests: The authors have read the journal's policy and have the following conflicts: Dr. Maruyama, Ms. Yasuda, Dr. Kuroda and Dr. Eto are employees of Ajinomoto Co., Inc. The authors declare no financial conflict of interest was present regarding the results or interpretation of the reported experiments. Dr. Eto has applied for a patent on a kokumi-imparting agent (WO2007/055393). This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Figures
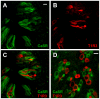
Figure 1. Taste cells express CaSR.
(A) RT-PCR for CaSR expression in taste bud-enriched circumvallate (cv), foliate (foli) and non-taste bud (nt) lingual epithelium. nc - negative control (lacking template); M - molecular standard. (B–E) Immunostaining for CaSR in taste buds. CaSR immunofluorescence is seen in most circumvallate (B), foliate (C), palate (D) and fungiform (E) taste buds. Immunofluorescent images (green) were superimposed on DIC images. (F) Validating the anti-CaSR antibody. The CaSR antiserum was preabsorbed with an excess of antigen peptides. The circumvallate sections reacted with preabsorbed and non-absorbed antibodies and were processed simultaneously. Images were taken under the same illumination conditions and detector settings. Scale bars 20 µm.
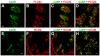
Figure 2. Confocal images showing colocalization of CaSR and the taste cell markers in taste cells from mouse circumvallate papillae.
(A–C) A longitudinal section of a circumvallate taste bud immunostained with antibodies against CaSR (A) and PLCβ2 (B). (C) Overlay of A and B. (D) A transverse section of a circumvallate taste bud immunostained with antibodies against CaSR (green) and PLCβ2 (red). (E–G) A longitudinal section of a circumvallate taste bud immunostained with antibodies against CaSR (E) and NCAM (F). (G) Overlay of E and F. (H) A transverse section of a circumvallate taste bud immunostained with antibodies against CaSR (green) and NCAM (red). Scale bars 20 µm. Arrowheads indicate double-labeled cells.
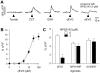
Figure 3. Taste cell responses (ΔCa2+) evoked by kokumi stimuli, recorded in a slice preparation of the mouse circumvallate papilla.
(A) Taste cells were stimulated sequentially with three kokumi substances, cinacalcet (CCT, 10 µM), glutathione (GSH, 100 µM) and γ-glutamyl-valinyl-glycine (γEVG, 100 µM), as well as γEVG + NPS2143 (3 µM), a CaSR antagonist. Arrowheads below traces indicate the stimulation. (B) Concentration-response relationship for γEVG (mean ± SE; n = 4 cells). (C) Taste responses elicited by γEVG (100 µM) were inhibited by the CaSR antagonist, NPS2143 (3 µM), but the umami (MPG 100 mM + IMP 1 mM) and the sweet (SC45647, 10 µM) responses were unaffected. Mean amplitudes of γEVG-, MPG + IMP-, and SC45647-induced responses in the presence or absence of 3 µM NPS2143 are shown (mean ± SE; *_p_≤0.05, n = 4 cells). Raw traces are shown in A.
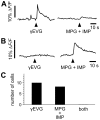
Figure 4. Ca2+ response elicited by γEVG involves intracellular Ca2+ stores and phospholipase C.
(A) γEVG (100 µM) was focally applied in medium containing Ca2+ (left trace) or in the absence of Ca2+ (Ca-free medium with 0.2 mM EGTA; right trace). (B) Responses elicited by depolarization (bath-applied KCl, 50 mM) and influx of Ca2+ through voltage-dependent Ca2+ channels were abolished in the absence of extracellular Ca2+. (C) Mean amplitudes of responses in the presence or absence of Ca2+ in the medium (mean ± SE; *_p_≤0.05, n = 4 cells). (D) Responses to γEVG inhibited by U73122 (10 µM). (E) Mean amplitudes of the responses in the presence or absence of U73122 (mean ± SE; *_p_≤0.05, n = 4 cells).
Figure 5. γEVG-responding taste cells are different from MPG + IMP-responding cells.
(A) Taste cell responses were recorded in lingual slice preparations of mouse circumvallate papilla. Preparations were sequentially stimulated with γEVG (100 µM) and MPG (100 mM) + IMP (1 mM). The traces show superimposed responses from γEVG-responding cells (A) and MPG + IMP-responding cells (B). Responses to γEVG were only observed in cells lacking response to MPG + IMP. (C) We recorded from 132 Calcium Green-loaded taste cells in 16 lingual slices. Ten dye-loaded cells were γEVG-responding cells, while MPG + IMP stimulation evoked responses in 8 out of 132 cells. We did not identify cells that responded to both substances.
Figure 6. CaSR is found in distinct cells that do not express an umami/sweet receptor subunit.
(A–C) A longitudinal section of a circumvallate taste bud immunostained with antibodies against CaSR (A) and T1R3 (B). (C) Overlay of A and B. (D) A transverse section of a circumvallate taste bud immunostained with antibodies against CaSR (green) and T1R3 (red). Scale bars 20 µm.
Similar articles
- Involvement of the calcium-sensing receptor in human taste perception.
Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y. Ohsu T, et al. J Biol Chem. 2010 Jan 8;285(2):1016-22. doi: 10.1074/jbc.M109.029165. Epub 2009 Nov 5. J Biol Chem. 2010. PMID: 19892707 Free PMC article. - Enhancement of allyl isothiocyanate-evoked responses of mouse trigeminal ganglion cells by the kokumi substance γ-glutamyl-valyl-glycine (γ-EVG) through activation of the calcium-sensing receptor (CaSR).
Akiyama T, Curtis E, Carstens MI, Carstens E. Akiyama T, et al. Physiol Behav. 2023 Mar 1;260:114063. doi: 10.1016/j.physbeh.2022.114063. Epub 2022 Dec 21. Physiol Behav. 2023. PMID: 36563734 Free PMC article. - Oral expressions and functional analyses of the extracellular calcium-sensing receptor (CaSR) in chicken.
Omori H, Kawabata Y, Yoshida Y, Nagamoto Y, Kawabata F, Nishimura S, Tabata S. Omori H, et al. Sci Rep. 2022 Oct 22;12(1):17762. doi: 10.1038/s41598-022-22512-6. Sci Rep. 2022. PMID: 36273034 Free PMC article. - Taste information derived from T1R-expressing taste cells in mice.
Yoshida R, Ninomiya Y. Yoshida R, et al. Biochem J. 2016 Mar 1;473(5):525-36. doi: 10.1042/BJ20151015. Biochem J. 2016. PMID: 26912569 Review. - Current progress in kokumi-active peptides, evaluation and preparation methods: a review.
Li Q, Zhang L, Lametsch R. Li Q, et al. Crit Rev Food Sci Nutr. 2022;62(5):1230-1241. doi: 10.1080/10408398.2020.1837726. Epub 2020 Oct 26. Crit Rev Food Sci Nutr. 2022. PMID: 33103468 Review.
Cited by
- Genetics of taste receptors.
Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, Nelson TM. Bachmanov AA, et al. Curr Pharm Des. 2014;20(16):2669-83. doi: 10.2174/13816128113199990566. Curr Pharm Des. 2014. PMID: 23886383 Free PMC article. Review. - Chemical and Sensory Characteristics of Soy Sauce: A Review.
Diez-Simon C, Eichelsheim C, Mumm R, Hall RD. Diez-Simon C, et al. J Agric Food Chem. 2020 Oct 21;68(42):11612-11630. doi: 10.1021/acs.jafc.0c04274. Epub 2020 Sep 17. J Agric Food Chem. 2020. PMID: 32880168 Free PMC article. Review. - Interkingdom Detection of Bacterial Quorum-Sensing Molecules by Mammalian Taste Receptors.
Kouakou YI, Lee RJ. Kouakou YI, et al. Microorganisms. 2023 May 16;11(5):1295. doi: 10.3390/microorganisms11051295. Microorganisms. 2023. PMID: 37317269 Free PMC article. Review. - Distribution and localization of porcine calcium sensing receptor in different tissues of weaned piglets1.
Zhao X, Schindell B, Li W, Ni L, Liu S, Wijerathne CUB, Gong J, Nyachoti CM, O K, Yang C. Zhao X, et al. J Anim Sci. 2019 May 30;97(6):2402-2413. doi: 10.1093/jas/skz096. J Anim Sci. 2019. PMID: 30887022 Free PMC article. - pH effects on plant calcium fluxes: lessons from acidification-mediated calcium elevation induced by the γ-glutamyl-leucine dipeptide identified from Phytophthora infestans.
Westphal L, Strehmel N, Eschen-Lippold L, Bauer N, Westermann B, Rosahl S, Scheel D, Lee J. Westphal L, et al. Sci Rep. 2019 Mar 18;9(1):4733. doi: 10.1038/s41598-019-41276-0. Sci Rep. 2019. PMID: 30894659 Free PMC article.
References
- Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, et al. Cloning and characterization of an extracellular Ca2+−sensing receptor from bovine parathyroid. Nature. 1993;366:575–580. - PubMed
- Chattopadhyay N, Vassilev PM, Brown EM. Calcium-sensing receptor: roles in and beyond systemic calcium homeostasis. Biol Chem. 1997;378:759–768. - PubMed
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. - PubMed
- Bystrova MF, Romanov RA, Rogachevskaja OA, Churbanov GD, Kolesnikov SS. Functional expression of the extracellular-Ca2+-sensing receptor in mouse taste cells. J Cell Sci. 2010;123:972–982. - PubMed
- San Gabriel A, Uneyama H, Maekawa T, Torii K. The calcium-sensing receptor in taste tissue. Biochem Biophys Res Commun. 2009;378:414–418. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous