Tuning synaptic transmission in the hippocampus by stress: the CRH system - PubMed (original) (raw)
Tuning synaptic transmission in the hippocampus by stress: the CRH system
Yuncai Chen et al. Front Cell Neurosci. 2012.
Abstract
To enhance survival, an organism needs to remember-and learn from-threatening or stressful events. This fact necessitates the presence of mechanisms by which stress can influence synaptic transmission in brain regions, such as hippocampus, that subserve learning and memory. A major focus of this series of monographs is on the role and actions of adrenal-derived hormones, corticosteroids, and of brain-derived neurotransmitters, on synaptic function in the stressed hippocampus. Here we focus on the contribution of hippocampus-intrinsic, stress-activated CRH-CRH receptor signaling to the function and structure of hippocampal synapses. Corticotropin-releasing hormone (CRH) is expressed in interneurons of adult hippocampus, and is released from axon terminals during stress. The peptide exerts time- and dose-dependent effects on learning and memory via modulation of synaptic function and plasticity. Whereas physiological levels of CRH, acting over seconds to minutes, augment memory processes, exposure to presumed severe-stress levels of the peptide results in spine retraction and loss of synapses over more protracted time-frames. Loss of dendritic spines (and hence of synapses) takes place through actin cytoskeleton collapse downstream of CRHR(1) receptors that reside within excitatory synapses on spine heads. Chronic exposure to stress levels of CRH may promote dying-back (atrophy) of spine-carrying dendrites. Thus, the acute effects of CRH may contribute to stress-induced adaptive mechanisms, whereas chronic or excessive exposure to the peptide may promote learning problems and premature cognitive decline.
Keywords: CRF; CRFR1; CRH receptor; corticotropin-releasing factor; hippocampus; long-term potentiation; neurotransmission; volume transmission.
Figures
Figure 1
CRH is expressed in interneurons within the hippocampal pyramidal cell layer. (A) CRH-immunoreactive (ir) neuronal somata in the pyramidal cell layer of area CA1. (B,C) All CRH-ir neurons in the pyramidal cell layer in CA1 area are GABAergic interneurons: they co-express the GABA synthesizing enzyme glutamic acid decarboxylase (GAD)-67 at both mRNA and protein levels, using immunocytochemistry coupled with in situ hybridization (GAD67, blue), and dual-labeled immunocytochemistry (CRH, brown; GAD67, black blue), respectively. Arrows indicate the dual-labeled neurons. (D–F) Many CRH-ir neurons in the hippocampus co-express parvalbumin (PV); none co-express calbindin D-28k (CB) or cholecystokinin (CCK). Solid arrows indicate the dual-labeled neurons, and empty arrows denote single-labeled CRH neurons. Abbreviations: so, stratum oriens; sp, stratum pyramidale; sr, stratum radiatum; slm, stratum lacunosum-moleculare. Scale bars = 75 μm (A,B,F), 25 μm (C), and 50 μm (D,E). Reproduced, with permission, from Chen et al. (2004b) (B) and Yan et al. (1998) (D,E).
Figure 2
CRH is located in GABAergic axonal terminals. (A) CRH-ir axon terminals form dense perisomatic baskets (arrows) around pyramidal cells in hippocampal area CA3. (B) CRH immunoreactivity localizes to pre-synaptic terminals, demonstrated by dual-labeled immunocytochemistry for CRH and for the pre-synaptic marker syntaxin. (C,D) CRH-containing terminals are GABAergic boutons as indicated by overlapping immunoreactivities of CRH and GAD65 (C) and of CRH and the GABA synaptic vesicular transporter VGAT (D) in axon terminals. (E) CRH-ir axon terminals (at) form axosomatic symmetric synapses (arrowhead) with pyramidal cell body (soma). (F) CRH-ir axon terminals form axodendritic asymmetric synapses (arrowhead) with dendrites (dend). Empty arrow indicates a bouton containing CRH-negative vesicles. (G) Electron micrograph demonstrating CRH-ir gold particles (arrow) within the dendrite, but not within vesicles. Scale bars = 50 μm (A), 10 μm in (D) (25 μm for B and 12.5 μm for C), and 0.2 μm (E–G). Reproduced, with permission, from Chen et al. (2004b) (B–D) and Yan et al. (1998) (E).
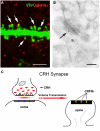
Figure 3
The post-synaptic location of the CRH receptor CRFR1 on dendritic spines. (A) The CRH receptor CRFR1 (red) is located at the dendritic spine heads, as shown by confocal microscopy of CA3 pyramidal neuronal dendrite (green) from a YFP-expressing mouse. Arrows denote spine heads expressing the receptor. (B) Electron micrograph showing CRFR1-immunogold particles (arrow) concentrated at the post-synaptic density (PSD) of an asymmetric (excitatory) synapse on a dendritic spine (s) in stratum oriens of hippocampal area CA3. Very few gold grains were observed elsewhere. (C) A cartoon depicting the concept of the mis-matched CRH synapse: pre-synaptic elements (axon terminal and boutons) are GABAergic (Figure 1), whereas post-synaptic elements on dendritic spines are consistent with excitatory synapses (Figure 3A). CRH that is released from inhibitory pre-synaptic elements may migrate via volume transmission and act on the CRFR1 receptor at relatively distant excitatory post-synaptic sites. Scale bars = 3 μm (A), 0.1 μm (B). Reproduced, with permission, from Chen et al. (2010) (A), (2004b) (B).
Figure 4
Stress induces rapid release of endogenous CRH into the extracellular space within hippocampus. (A,B) Visualization of stress-induced release of endogenous CRH into the extracellular space (neuropil) in area CA3. Under control conditions (A1,A2), there is little CRH-immunoreactivity outside of the cell bodies and terminals. In contrast, after a 30-min stress (B1,B2), immunoreactive peptide is visible within the neuropil of the pyramidal cell layer and adjacent regions. See Chen et al. (2004b) for experimental details. Frames in (A1,B1) denote areas magnified in (A2,B2), respectively, demonstrating the presence of CRH-immunoreactivity in the extracellular space. (C) Semi-quantitative analysis of CRH-immunoreactivity in presumed extracellular spaces of area CA3 (n = 5 animals, *P < 0.01). (D) Stress-evoked release of endogenous CRH (red) into the extracellular space. NeuN (green) indicates CA3 pyramidal cells. Confocal image from a P18 rat sacrificed after a 30-min stress. Abbreviations: so, stratum oriens; sp, stratum pyramidale; sl, stratum lucidum. Scale bars = 60 μm in (B1,A1), 20 μm in (B2,A2, and D). Reproduced, with permission, from Chen et al. (2004b) (D).
Similar articles
- Converging, Synergistic Actions of Multiple Stress Hormones Mediate Enduring Memory Impairments after Acute Simultaneous Stresses.
Chen Y, Molet J, Lauterborn JC, Trieu BH, Bolton JL, Patterson KP, Gall CM, Lynch G, Baram TZ. Chen Y, et al. J Neurosci. 2016 Nov 2;36(44):11295-11307. doi: 10.1523/JNEUROSCI.2542-16.2016. J Neurosci. 2016. PMID: 27807170 Free PMC article. - NMDA receptor activation and calpain contribute to disruption of dendritic spines by the stress neuropeptide CRH.
Andres AL, Regev L, Phi L, Seese RR, Chen Y, Gall CM, Baram TZ. Andres AL, et al. J Neurosci. 2013 Oct 23;33(43):16945-60. doi: 10.1523/JNEUROSCI.1445-13.2013. J Neurosci. 2013. PMID: 24155300 Free PMC article. - Correlated memory defects and hippocampal dendritic spine loss after acute stress involve corticotropin-releasing hormone signaling.
Chen Y, Rex CS, Rice CJ, Dubé CM, Gall CM, Lynch G, Baram TZ. Chen Y, et al. Proc Natl Acad Sci U S A. 2010 Jul 20;107(29):13123-8. doi: 10.1073/pnas.1003825107. Epub 2010 Jul 6. Proc Natl Acad Sci U S A. 2010. PMID: 20615973 Free PMC article. - Stress and anxiety in schizophrenia and depression: glucocorticoids, corticotropin-releasing hormone and synapse regression.
Bennett A O MR. Bennett A O MR. Aust N Z J Psychiatry. 2008 Dec;42(12):995-1002. doi: 10.1080/00048670802512073. Aust N Z J Psychiatry. 2008. PMID: 19016087 Review. - The stress system in the human brain in depression and neurodegeneration.
Swaab DF, Bao AM, Lucassen PJ. Swaab DF, et al. Ageing Res Rev. 2005 May;4(2):141-94. doi: 10.1016/j.arr.2005.03.003. Ageing Res Rev. 2005. PMID: 15996533 Review.
Cited by
- Environmental factors in autism.
Grabrucker AM. Grabrucker AM. Front Psychiatry. 2013 Jan 18;3:118. doi: 10.3389/fpsyt.2012.00118. eCollection 2012. Front Psychiatry. 2013. PMID: 23346059 Free PMC article. - Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex.
Rodriguez GA, Burns MP, Weeber EJ, Rebeck GW. Rodriguez GA, et al. Learn Mem. 2013 Apr 16;20(5):256-66. doi: 10.1101/lm.030031.112. Learn Mem. 2013. PMID: 23592036 Free PMC article. - Hippocampal interneurons are direct targets for circulating glucocorticoids.
Kraus KL, Chordia AP, Drake AW, Herman JP, Danzer SC. Kraus KL, et al. J Comp Neurol. 2022 Aug;530(12):2100-2112. doi: 10.1002/cne.25322. Epub 2022 Apr 9. J Comp Neurol. 2022. PMID: 35397117 Free PMC article. - Social rank-associated stress vulnerability predisposes individuals to cocaine attraction.
Yanovich C, Kirby ML, Michaelevski I, Yadid G, Pinhasov A. Yanovich C, et al. Sci Rep. 2018 Jan 29;8(1):1759. doi: 10.1038/s41598-018-19816-x. Sci Rep. 2018. PMID: 29379100 Free PMC article. - Cortical Thinning and Neuropsychiatric Outcomes in Children Exposed to Prenatal Adversity: A Role for Placental CRH?
Sandman CA, Curran MM, Davis EP, Glynn LM, Head K, Baram TZ. Sandman CA, et al. Am J Psychiatry. 2018 May 1;175(5):471-479. doi: 10.1176/appi.ajp.2017.16121433. Epub 2018 Mar 2. Am J Psychiatry. 2018. PMID: 29495899 Free PMC article.
References
- Agnati L. F., Bjelke B., Fuxe K. (1995). Volume versus wiring transmission in the brain: a new theoretical frame for neuropsychopharmacology. Med. Res. Rev. 15, 33–45 - PubMed