Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography - PubMed (original) (raw)
Comparative Study
. 2012 May 2;104(9):700-8.
doi: 10.1093/jnci/djs188. Epub 2012 Apr 18.
Affiliations
- PMID: 22517990
- PMCID: PMC3341309
- DOI: 10.1093/jnci/djs188
Comparative Study
Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography
Henri J L M Timmers et al. J Natl Cancer Inst. 2012.
Abstract
Background: Pheochromocytomas and paragangliomas (PPGLs) are rare tumors of the adrenal medulla and extra-adrenal sympathetic chromaffin tissues; their anatomical and functional imaging are critical to guiding treatment decisions. This study aimed to compare the sensitivity and specificity of (18)F-fluorodeoxyglucose positron emission tomography with computed tomography ((18)F-FDG PET/CT) for tumor localization and staging of PPGLs with that of conventional imaging by [(123)I]-metaiodobenzylguanidine single photon emission CT ((123)I-MIBG SPECT), CT, and magnetic resonance imaging (MRI).
Methods: A total of 216 patients (106 men, 110 women, aged 45.2 ± 14.9 years) with suspected PPGL underwent CT or MRI, (18)F-FDG PET/CT, and (123)I-MIBG SPECT/CT. Sensitivity and specificity were measured as endpoints and compared by the McNemar test, using two-sided P values only.
Results: Sixty (28%) of patients had nonmetastatic PPGL, 95 (44%) had metastatic PPGL, and 61 (28%) were PPGL negative. For nonmetastatic tumors, the sensitivity of (18)F-FDG was similar to that of (123)I-MIBG but less than that of CT/MRI (sensitivity of (18)F-FDG = 76.8%; of (123)I-MIBG = 75.0%; of CT/MRI = 95.7%; (18)F-FDG vs (123)I-MIBG: difference = 1.8%, 95% confidence interval [CI] = -14.8% to 14.8%, P = .210; (18)F-FDG vs CT/MRI: difference = 18.9%, 95% CI = 9.4% to 28.3%, P < .001). The specificity was 90.2% for (18)F-FDG, 91.8% for (123)I-MIBG, and 90.2% for CT/MRI. (18)F-FDG uptake was higher in succinate dehydrogenase complex- and von Hippel-Lindau syndrome-related tumors than in multiple endocrine neoplasia type 2 (MEN2) related tumors. For metastases, sensitivity was greater for (18)F-FDG and CT/MRI than for (123)I-MIBG (sensitivity of (18)F-FDG = 82.5%; of (123)I-MIBG = 50.0%; of CT/MRI = 74.4%; (18)F-FDG vs (123)I-MIBG: difference = 32.5%, 95% CI = 22.3% to 42.5%, P < .001; CT/MRI vs (123)I-MIBG: difference = 24.4%, 95% CI = 11.3% to 31.6%, P < .001). For bone metastases, (18)F-FDG was more sensitive than CT/MRI (sensitivity of (18)F-FDG = 93.7%; of CT/MRI = 76.7%; difference = 17.0%, 95% CI = 4.9% to 28.5%, P = .013).
Conclusions: Compared with (123)I-MIBG SPECT and CT/MRI, both considered gold standards for PPGL imaging, metastases were better detected by (18)F-FDG PET. (18)F-FDG PET provides a high specificity in patients with a biochemically established diagnosis of PPGL.
Figures
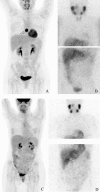
Figure 1
18F-fluorodeoxyglucose positron emission tomography (18F-FDG) and [123I]-metaiodobenzylguanidine (123I-MIBG) images of patients with succinate dehydrogenase subunit B (SDHB) mutations and multiple endocrine neoplasia-2 (MEN2). A and B) Maximum intensity projected 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) (A) and [123I]-metaiodobenzylguanidine (123I-MIBG) scintigraphy (SPECT) (B) of an SDHB patient with a paraganglioma of the mediastinum (Table 1: SDHB, F36): lesion marked by arrow. C and D) Maximum intensity projected 18F-FDG PET (C) and 123I-MIBG scintigraphy (D) of an MEN2 patient with a right adrenal pheochromocytoma (Table 1: MEN2, F40): lesion marked by arrow.
Figure 2
18F-fluorodeoxyglucose positron emission tomography standardized uptake value (SUV) in pheochromocytoma and paraganglioma (PPGL) vs normal adrenal glands. Box and whiskers diagrams of maximum SUVs are statistically significantly higher for adrenal and extra-adrenal PPGLs than for normal adrenal glands in patients without PPGL and contralateral adrenal or extra-adrenal PPGL (boxes indicate 25th and 75th percentile, solid lines indicate median, and whiskers indicate range) (Tukey–Kramer test, two-sided).
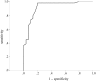
Figure 3
Receiver operating curve for 18F-fluorodeoxyglucose positron emission tomography standardized uptake value (SUV). This curve was constructed from the SUVs of pheochromocytomas and normal adrenals in patients with nonmetastatic pheochromocytomas and paragangliomas.
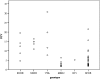
Figure 4
18F-fluorodeoxyglucose positron emission tomography standardized uptake value (SUV) in nonmetastatic pheochromocytoma and paraganglioma across genotypes. MEN2 = multiple endocrine neoplasia type 2, MN = plasma metaneprine, NF1 = neurofibromatosis type 1, NMN = plasma normetanephrine, ns = nonsignificant, SDHB/C/D/x= succinate dehydrogenase subunit B/C/D/x, SPOR = sporadic, SUVmax = maximum SUV, VHL = von Hippel–Lindau syndrome.
Similar articles
- 6-[F-18]Fluoro-L-dihydroxyphenylalanine positron emission tomography is superior to conventional imaging with (123)I-metaiodobenzylguanidine scintigraphy, computer tomography, and magnetic resonance imaging in localizing tumors causing catecholamine excess.
Fiebrich HB, Brouwers AH, Kerstens MN, Pijl ME, Kema IP, de Jong JR, Jager PL, Elsinga PH, Dierckx RA, van der Wal JE, Sluiter WJ, de Vries EG, Links TP. Fiebrich HB, et al. J Clin Endocrinol Metab. 2009 Oct;94(10):3922-30. doi: 10.1210/jc.2009-1054. Epub 2009 Jul 21. J Clin Endocrinol Metab. 2009. PMID: 19622618 - Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma.
Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Timmers HJ, et al. J Clin Endocrinol Metab. 2009 Dec;94(12):4757-67. doi: 10.1210/jc.2009-1248. Epub 2009 Oct 28. J Clin Endocrinol Metab. 2009. PMID: 19864450 Free PMC article. - The utility of 68Ga-DOTATATE PET/CT in localizing primary/metastatic pheochromocytoma and paraganglioma in children and adolescents - a single-center experience.
Jaiswal SK, Sarathi V, Malhotra G, Hira P, Shah R, Patil VA, Dalvi A, Prakash G, Lila AR, Shah NS, Bandgar T. Jaiswal SK, et al. J Pediatr Endocrinol Metab. 2020 Nov 12;34(1):109-119. doi: 10.1515/jpem-2020-0354. Print 2021 Jan 27. J Pediatr Endocrinol Metab. 2020. PMID: 33180042 Clinical Trial. - Imaging of pheochromocytoma and paraganglioma.
Brink I, Hoegerle S, Klisch J, Bley TA. Brink I, et al. Fam Cancer. 2005;4(1):61-8. doi: 10.1007/s10689-004-2155-y. Fam Cancer. 2005. PMID: 15883712 Review. - Current and future anatomical and functional imaging approaches to pheochromocytoma and paraganglioma.
Timmers HJ, Taieb D, Pacak K. Timmers HJ, et al. Horm Metab Res. 2012 May;44(5):367-72. doi: 10.1055/s-0031-1299712. Epub 2012 Mar 7. Horm Metab Res. 2012. PMID: 22399235 Free PMC article. Review.
Cited by
- Somatostatin Receptors and Analogs in Pheochromocytoma and Paraganglioma: Old Players in a New Precision Medicine World.
Patel M, Tena I, Jha A, Taieb D, Pacak K. Patel M, et al. Front Endocrinol (Lausanne). 2021 Mar 29;12:625312. doi: 10.3389/fendo.2021.625312. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 33854479 Free PMC article. Review. - The incremental benefit of functional imaging in pheochromocytoma/paraganglioma: a systematic review.
Brito JP, Asi N, Gionfriddo MR, Norman C, Leppin AL, Zeballos-Palacios C, Undavalli C, Wang Z, Domecq JP, Prustsky G, Elraiyah TA, Prokop LJ, Montori VM, Murad MH. Brito JP, et al. Endocrine. 2015 Sep;50(1):176-86. doi: 10.1007/s12020-015-0544-7. Epub 2015 Feb 6. Endocrine. 2015. PMID: 25663601 Review. - Management of phaeochromocytoma and paraganglioma in patients with germline SDHB pathogenic variants: an international expert Consensus statement.
Taïeb D, Nölting S, Perrier ND, Fassnacht M, Carrasquillo JA, Grossman AB, Clifton-Bligh R, Wanna GB, Schwam ZG, Amar L, Bourdeau I, Casey RT, Crona J, Deal CL, Del Rivero J, Duh QY, Eisenhofer G, Fojo T, Ghayee HK, Gimenez-Roqueplo AP, Gill AJ, Hicks R, Imperiale A, Jha A, Kerstens MN, de Krijger RR, Lacroix A, Lazurova I, Lin FI, Lussey-Lepoutre C, Maher ER, Mete O, Naruse M, Nilubol N, Robledo M, Sebag F, Shah NS, Tanabe A, Thompson GB, Timmers HJLM, Widimsky J, Young WJ Jr, Meuter L, Lenders JWM, Pacak K. Taïeb D, et al. Nat Rev Endocrinol. 2024 Mar;20(3):168-184. doi: 10.1038/s41574-023-00926-0. Epub 2023 Dec 14. Nat Rev Endocrinol. 2024. PMID: 38097671 Review. - Progress in the diagnosis and treatment of paraganglioma.
Wang B, Qiu J. Wang B, et al. Transl Cancer Res. 2019 Nov;8(7):2624-2635. doi: 10.21037/tcr.2019.10.11. Transl Cancer Res. 2019. PMID: 35117020 Free PMC article. Review. - Primary malignant tumors of the adrenal glands.
Almeida MQ, Bezerra-Neto JE, Mendonça BB, Latronico AC, Fragoso MCBV. Almeida MQ, et al. Clinics (Sao Paulo). 2018 Dec 10;73(suppl 1):e756s. doi: 10.6061/clinics/2018/e756s. Clinics (Sao Paulo). 2018. PMID: 30540124 Free PMC article. Review.
References
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665–675. - PubMed
- Neumann HP, Bausch B, McWhinney SR, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459–1466. - PubMed
- Neumann HP, Pawlu C, Peczkowska M, et al. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292(8):943–951. - PubMed
- Timmers HJ, Kozupa A, Eisenhofer G, et al. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with SDHB-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92(3):779–786. - PubMed
- Lenders JW, Pacak K, Walther MM, et al. Biochemical diagnosis of pheochromocytoma: which test is best? JAMA. 2002;287(11):1427–1434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical