Performance of the cobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae - PubMed (original) (raw)
Comparative Study
Performance of the cobas CT/NG test compared to the Aptima AC2 and Viper CTQ/GCQ assays for detection of Chlamydia trachomatis and Neisseria gonorrhoeae
Barbara Van Der Pol et al. J Clin Microbiol. 2012 Jul.
Abstract
The next-generation amplification test for Chlamydia trachomatis and Neisseria gonorrhoeae (Roche cobas 4800), a fully automated system, was compared head to head, using female samples, to Gen-Probe Aptima Combo 2 and BD ProbeTec using Viper. Endocervical swabs, female urine, and endocervical samples in liquid-based cytology medium were run on at least two of three platforms. A total of 4,316 samples were evaluated, and 281 chlamydial and 69 gonococcal infections were identified. Estimates of sensitivity and specificity were obtained relative to the patient infection standard (PIS) and using latent class analysis (LCA). Chlamydia sensitivity estimates ranged from 86.9 to 95.6% using PIS and 97.6 to 98% using LCA. Specificity was ≥ 99.6% for all sample types. Sensitivity ranged from 95.6 to 100% using PIS and 96.9 to 100% using LCA for the detection of gonococcal infections. Specificity for gonococcal infections was ≥ 99.8%. cobas 4800 performance was equivalent to the comparator assays (all P values, >0.05), and the fully automated system provides high laboratory efficiency.
Figures
Fig 1
Venn diagrams comparing C. trachomatis-positive results across three assays of endocervical swabs (A), liquid-based cytology (B), and urine specimens (C) obtained from female subjects. A total of 4,223 (endocervical swabs), 4,186 (liquid based cytology), and 4,254 (urine specimens) subjects had valid results from c4800, AC2, and CT/GCQ assays.
Fig 2
Venn diagrams comparing N. gonorrhoeae-positive results across three assays of endocervical swabs (A), liquid-based cytology (B), and urine specimens (C) obtained from female subjects. A total of 4,221 (endocervical swabs), 4,187 (liquid-based cytology), and 4,255 (urine specimens) subjects had valid results from c4800, AC2, and CT/GCQ assays.
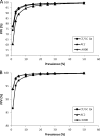
Fig 3
Hypothetical PPV based on changing population prevalence for C. trachomatis (A) and N. gonorrhoeae (B) results.
Similar articles
- Evaluation of the Performance of the Cobas CT/NG Test for Use on the Cobas 6800/8800 Systems for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Male and Female Urogenital Samples.
Van Der Pol B, Fife K, Taylor SN, Nye MB, Chavoustie SE, Eisenberg DL, Crane L, Hirsch G, Arcenas R, Marlowe EM. Van Der Pol B, et al. J Clin Microbiol. 2019 Mar 28;57(4):e01996-18. doi: 10.1128/JCM.01996-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30651389 Free PMC article. - Combined Testing for Chlamydia, Gonorrhea, and Trichomonas by Use of the BD Max CT/GC/TV Assay with Genitourinary Specimen Types.
Van Der Pol B, Williams JA, Fuller D, Taylor SN, Hook EW 3rd. Van Der Pol B, et al. J Clin Microbiol. 2016 Dec 28;55(1):155-164. doi: 10.1128/JCM.01766-16. Print 2017 Jan. J Clin Microbiol. 2016. PMID: 27795343 Free PMC article. - Performance of the Abbott RealTime CT/NG for detection of Chlamydia trachomatis and Neisseria gonorrhoeae.
Gaydos CA, Cartwright CP, Colaninno P, Welsch J, Holden J, Ho SY, Webb EM, Anderson C, Bertuzis R, Zhang L, Miller T, Leckie G, Abravaya K, Robinson J. Gaydos CA, et al. J Clin Microbiol. 2010 Sep;48(9):3236-43. doi: 10.1128/JCM.01019-10. Epub 2010 Jul 28. J Clin Microbiol. 2010. PMID: 20668135 Free PMC article. - Systematic review: noninvasive testing for Chlamydia trachomatis and Neisseria gonorrhoeae.
Cook RL, Hutchison SL, Østergaard L, Braithwaite RS, Ness RB. Cook RL, et al. Ann Intern Med. 2005 Jun 7;142(11):914-25. doi: 10.7326/0003-4819-142-11-200506070-00010. Ann Intern Med. 2005. PMID: 15941699 Review. - Cobas® 4800: a fully automated system for the detection of Chlamydia trachomatis and Neisseria gonorrhoeae.
Van Der Pol B. Van Der Pol B. Expert Rev Mol Diagn. 2013 Mar;13(2):131-40. doi: 10.1586/erm.12.141. Expert Rev Mol Diagn. 2013. PMID: 23477553 Review.
Cited by
- The new frontier of diagnostics: Molecular assays and their role in infection prevention and control.
Das S, Shibib DR, Vernon MO. Das S, et al. Am J Infect Control. 2017 Feb 1;45(2):158-169. doi: 10.1016/j.ajic.2016.08.005. Am J Infect Control. 2017. PMID: 28159066 Free PMC article. Review. - Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection.
Geisler WM, Lensing SY, Press CG, Hook EW 3rd. Geisler WM, et al. J Infect Dis. 2013 Jun 15;207(12):1850-6. doi: 10.1093/infdis/jit094. Epub 2013 Mar 7. J Infect Dis. 2013. PMID: 23470847 Free PMC article. - Diagnostic efficacy of a real time-PCR assay for Chlamydia trachomatis infection in infertile women in north India.
Dhawan B, Rawre J, Ghosh A, Malhotra N, Ahmed MM, Sreenivas V, Chaudhry R. Dhawan B, et al. Indian J Med Res. 2014 Aug;140(2):252-61. Indian J Med Res. 2014. PMID: 25297359 Free PMC article. - Evaluation of the Performance of the Cobas CT/NG Test for Use on the Cobas 6800/8800 Systems for Detection of Chlamydia trachomatis and Neisseria gonorrhoeae in Male and Female Urogenital Samples.
Van Der Pol B, Fife K, Taylor SN, Nye MB, Chavoustie SE, Eisenberg DL, Crane L, Hirsch G, Arcenas R, Marlowe EM. Van Der Pol B, et al. J Clin Microbiol. 2019 Mar 28;57(4):e01996-18. doi: 10.1128/JCM.01996-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30651389 Free PMC article. - Combined Testing for Chlamydia, Gonorrhea, and Trichomonas by Use of the BD Max CT/GC/TV Assay with Genitourinary Specimen Types.
Van Der Pol B, Williams JA, Fuller D, Taylor SN, Hook EW 3rd. Van Der Pol B, et al. J Clin Microbiol. 2016 Dec 28;55(1):155-164. doi: 10.1128/JCM.01766-16. Print 2017 Jan. J Clin Microbiol. 2016. PMID: 27795343 Free PMC article.
References
- Bjorkman J, Jonsson L, Nilsson P. 2007. Prevalence of the new genetic variant of Chlamydia trachomatis in Sodra Alvsborg County, Vastra Gotaland Region, Sweden. Euro Surveill. 12:E070614.4. - PubMed
- Castle P, et al. 2011. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the Athena study. Lancet Oncol. 12:880–890 - PubMed
- Centers for Disease Control and Prevention 2010. Laboratory diagnostic testing for Chlamydia trachomatis and Neisseria gonorrhoeae. American Public Health Laboratories, Centers for Disease Control and Prevention, Atlanta, GA: http://www.aphl.org/aphlprograms/infectious/std/documents/ctgclabguideli...
- Centers for Disease Control and Prevention 2010. Sexually transmitted diseases treatment guidelines, 2010. Morb. Mortal. Wkly. Rep. 59:1–112
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials