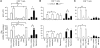CD4(+) T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease - PubMed (original) (raw)
. 2012 May;122(5):1816-31.
doi: 10.1172/JCI60862. Epub 2012 Apr 23.
Rossana G Iannitti, Silvia Bozza, Remi Beau, Andrea Casagrande, Carmen D'Angelo, Silvia Moretti, Cristina Cunha, Gloria Giovannini, Cristina Massi-Benedetti, Agostinho Carvalho, Louis Boon, Jean-Paul Latgé, Luigina Romani
Affiliations
- PMID: 22523066
- PMCID: PMC3336987
- DOI: 10.1172/JCI60862
CD4(+) T cell vaccination overcomes defective cross-presentation of fungal antigens in a mouse model of chronic granulomatous disease
Antonella De Luca et al. J Clin Invest. 2012 May.
Abstract
Aspergillus fumigatus is a model fungal pathogen and a common cause of infection in individuals with the primary immunodeficiency chronic granulomatous disease (CGD). Although primarily considered a deficiency of innate immunity, CGD is also linked to dysfunctional T cell reactivity. Both CD4(+) and CD8(+) T cells mediate vaccine-induced protection from experimental aspergillosis, but the molecular mechanisms leading to the generation of protective immunity and whether these mechanisms are dysregulated in individuals with CGD have not been determined. Here, we show that activation of either T cell subset in a mouse model of CGD is contingent upon the nature of the fungal vaccine, the involvement of distinct innate receptor signaling pathways, and the mode of antigen routing and presentation in DCs. Aspergillus conidia activated CD8(+) T cells upon sorting to the Rab14(+) endosomal compartment required for alternative MHC class I presentation. Cross-priming of CD8(+) T cells failed to occur in mice with CGD due to defective DC endosomal alkalinization and autophagy. However, long-lasting antifungal protection and disease control were successfully achieved upon vaccination with purified fungal antigens that activated CD4(+) T cells through the endosome/lysosome pathway. Our study thus indicates that distinct intracellular pathways are exploited for the priming of CD4(+) and CD8(+) T cells to A. fumigatus and suggests that CD4(+) T cell vaccination may be able to overcome defective antifungal CD8(+) T cell memory in individuals with CGD.
Figures
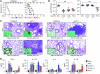
Figure 1. p47phox–/– mice fail to develop vaccine-induced resistance to A. fumigatus conidia.
Mice (6/group) received A. fumigatus conidia i.n. or s.c 14 days before reinfection with Aspergillus conidia i.n. Pep1p was given i.n. with CpG 14, 7, and 3 days before reinfection. Mice were given cyclophosphamide a day before reinfection. Vaccine-induced resistance was assessed in terms of (A) survival (%); (B) fungal growth (log10 CFU ± SEM); (C) lung histology (PAS staining and Gomori staining in the insets, visualized, respectively, with an original magnification of ×200 and ×1,000); and (D) cytokine production (ELISA) by purified lung T cells cultured in vitro with conidia- or Pep1p-pulsed DCs from the corresponding naive mice. In C, values represent percentages of polymorphonuclear (PMN) or mononuclear (MNC) cells in the BAL. Assays were done at 3 days after infection. Data are pooled or representative (histology) of 3 independent experiments.*P < 0.05, **P < 0.01, ***P < 0.001, vaccinated versus unvaccinated (None) mice. Scale bars: 200 μm.
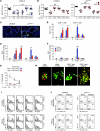
Figure 2. p47phox–/– mice fail to develop MHC class I–restricted CD8+ T cell responses to Aspergillus conidia.
Mice (6/group) received conidia or Pep1p plus CpG as described for Figure 1 and were concomitantly treated with the indicated antibodies or an isotype-matched control antibody (None). Fungal growth (log10 CFU ± SEM) in conidia-vaccinated (A) or Pep1p-vaccinated (B) mice at 3 days after reinfection. Control (Ct), infected, unvaccinated mice. Pooled data from 3 experiments are shown. (C) Lung immunohistochemistry 3 days after reinfection. Cell surface markers were Alexa Fluor 488–anti-CD4 and Alexa Fluor 647–anti-CD8 antibody. Cell nuclei were stained with DAPI (blue). Representative pictures (of 3 experiments) were taken with an original magnification of ×200. (D) Proliferation of CD4+ and CD8+ T cells purified from lungs 1 week after a primary i.n. infection. DNA synthesis was measured by 3H-thymidine uptake after 72 hours coculture with conidia- or Pep1p-pulsed DCs from the corresponding naive mice. Ct, T cells alone. Relative expression of (E) Ifng and (F) Prf1 by RT-PCR in CD4+ and CD8+ T cells exposed to conidia- or Pep1p-pulsed DCs for 24 hours. (G) Cytolytic activity of CD8+ T cells, obtained as in D, against conidia-pulsed DCs at different E/T ratios. Shown is the percentage of specific cytotoxic activity determined by a standard 4-hour 51Cr-release assay. (H) Conidiocidal activity of culture supernatants from CD8+ T cells exposed as in D (visualized with an original magnification of ×400). Activation/memory marker expression (I) and intracellular cytokine staining (J) by lung CD8+ and CD4+ T cells purified from conidia- or Pep1p-vaccinated mice, respectively, 3 days after reinfection. Histograms were generated from pooled samples of 6 mice/group. Representative histograms from a single experiment are shown. Values are the percentages of positive cells. *P < 0.05, **P < 0.01, ***P < 0.001 treated versus untreated mice (A and B) and pulsed DC–stimulated versus unstimulated cells (None) (D–G).
Figure 3. CD4+ or CD8+ T cell vaccination to A. fumigatus requires distinct TLR signaling.
Dectin-1, TLR-, MyD88-, or TRIF-deficient mice were administered A. fumigatus conidia or the protective recombinant fungal antigens (Pep1p, Gel1p, or Crf1p) plus CpG and assessed for resistance to reinfection in terms of (A) survival (%), (B) fungal growth (log10 CFU ± SEM), and (C) proliferation of CD4+ or CD8+ T cells, purified from Pep1p- or conidia-vaccinated mice, respectively, at 3 days after infection, and cultured in vitro with conidia- or Pep1p-pulsed DCs from the corresponding naive mice. DNA synthesis was measured by 3H-thymidine uptake after 72 hours coculture. *P < 0.05, **P < 0.01, ***P < 0.001, vaccinated versus unvaccinated (None) mice. ND, not done. Data are representative of 3 experiments.
Figure 4. CD4+ and CD8+ T cells are activated through distinct intracellular antigen presentation pathways.
CD8+ or CD4+ T cells were purified from lungs of C57BL/6 or p47phox–/– mice a week after the intranasal infection and exposed to conidia-pulsed (A) or Pep1p-pulsed (B) DCs purified from lungs of the corresponding naive mice. Prior to the 2-hour pulsing with conidia or Pep1p, DCs were exposed to the indicated antigen presentation pathway inhibitors for 120 minutes. (C) CD8+ T cells, purified as in A, were exposed to DCs and/or intact or apoptotic (Apo) alveolar macrophages (AM), purified from lungs of the corresponding naive mice. AMs were pulsed to live conidia before the induction of apoptosis with LPS plus ATP. Cells were assessed for proliferation and Ifng expression by RT-PCR, 72 hours after the coculture. DNA synthesis was measured by 3H-thymidine uptake. Data are from 4 independent experiments.*P < 0.05, **P < 0.01, inhibitors versus no inhibitors; and DC-exposed versus T cells alone (Ct).
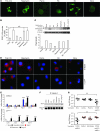
Figure 5. Distinct intracellular routing of Aspergillus conidia and soluble fungal antigens in DCs and its subversion in the absence of NADPH.
Immunofluorescence imaging of purified DCs from lungs of C57BL/6 (A) or p47phox–/– (C) mice after in vitro exposure to GFP-conidia or Alexa Fluor 488–Pep1p at 37°C for 2 hours and chasing for 15 and 45 minutes. (B and D) DCs were preincubated with 5 mM 3-MA or 5 μg/ml chloroquine for 30 minutes prior to exposure to GFP-conidia or to Alexa Fluor 488–Pep1p. Formaldehyde-fixed DCs were incubated with primary antibodies against MR, Rab5, Rab7, Rab9, Rab14, and Lamp1, followed by secondary anti-rabbit IgG–TRITC antibody. Nuclei were counterstained with DAPI. Images were acquired using a fluorescence microscope (BX51) with an original magnification of ×1,000 and analySIS image processing software. Shown are merged images of DCs (a single cell is magnified in the inset visualized with an original magnification of ×1,000) pulsed with GFP-conidia or fungal antigens (green) and red-stained for each endosomal compartment. Shown are representative data from 4 independent experiments. For quantitative analysis of co-localization, see Supplemental Table 1.
Figure 6. CD11b+ p47phox–/– DCs efficiently present fungal antigens.
(A and B) Flow cytometry of phagocytosis of live GFP-expressing conidia by lung DCs purified from uninfected mice. Values are percentages of positive cells on T and B cell–depleted lung cells. Phagocytosis was quantified via phase contrast and fluorescence microscopy at ×1,000 magnification (shown are representative microscopy images of 2 independent experiments). (C) Mice were infected i.n. with GFP-expressing A. fumigatus conidia, and the numbers of GFP+CD11c+ cells were assessed by flow cytometry at 3 days after infection. Values are percentages of positive cells on gated CD11c+ cells. DCs purified from lung of naive mice were unpulsed (None) or pulsed with live Aspergillus conidia or Pep1p for (D) 24 hours prior to being assessed for cytokine gene expression by RT-PCR and (E) 2 hours before the assessment of pERK44/42 phosphorylation by immunoblot analysis. Shown is a representative Western blot of 2 independent experiments and corresponding pixel density ratio normalized against β-tubulin (β-tub). (F and G) Adoptive transfer of C57BL/6 or p47phox–/– lung DCs pulsed with conidia or Pep1p into C57BL/6 mice. DCs were adoptively transferred by i.p. injection twice, a week apart, before the i.n. infection. Fungal growth in the lungs (F, log10 CFU ± SEM, representative of at least 3 independent experiments) and histology (G, PAS staining) were determined 3 days after infection. *P < 0.05, **P < 0.01, ***P < 0.001, pulsed versus unpulsed DCs (D) and mice receiving or not (None) DCs (F). Scale bars: 200 μm.
Figure 7. Autophagy restores defective cross-presentation of conidia in p47phox–/– mice.
(A) Fluorescence images of EGFP-LC3 transiently transfected RAW 264.7 cells exposed to A. fumigatus swollen (SC) or resting (RC) conidia, Pep1p, rapamycin, or poly(I:C) for 4 hours. Original magnification, ×1,000. (B) Number of RAW 264.7 cells with EGFP-LC3 punctae (mean ± SEM, determined by fluorescence microscopy) (n = 2). (C) Cell lysates of EGFP-LC3–transfected RAW 264.7 cells were probed with anti-GFP antibody followed by IgG-HRP secondary antibody. Normalization was performed on mouse β-tubulin. Quantification was obtained by densitometry image analysis using Image Lab 3.1.1 software. (D) Autophagy on purified lung DCs stimulated as above and incubated with anti-LC3 antibody followed by PE secondary antibody. Representative images (original magnification, ×400) are shown. DAPI was used to detect nuclei. (E) Autophagy gene expression by RT-PCR in lung DCs stimulated as above. None, unstimulated cells. Data are representative of 2 experiments. (F) Cell lysates from EGFP-LC3–transfected RAW 264.7 cells stimulated as in A were subjected to immunoprecipitation with antibody to Beclin-1. Immunoprecipitates were probed with polyclonal antibody to TRIF. Data are representative of 2 experiments. (G) p47phox–/– mice were vaccinated with Aspergillus conidia and poly(I:C) under the condition of Becn1 inhibition by siRNA administration. Western blotting of lung cells confirmed that Beclin-1 protein decreases upon siBecn1 administration. Fungal growth (log10 CFU ± SEM) in the lungs was assessed 3 days after infection. Data are representative of 2 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, conidia + poly(I:C)–vaccinated versus unvaccinated (None) mice and with and without siBecn1.
Figure 8. Distinct pathways of intracellular antigen routing in CD4+ and CD8+ T cell vaccination to Aspergillus fumigatus.
The activation of CD4+ and CD8+ T cells is contingent upon the nature of the fungal vaccine, the involvement of distinct innate receptor signaling pathways, and the mode of antigen routing and presentation in DCs. Specifically, both soluble fungal antigens and phagocytosed conidia are routed to the endosome/lysosome compartment that is apparently required for adequate processing and presentation of antigens for CD4+ T cell activation. At variance with soluble antigens, conidia are diverted from the early endosomes to the Rab14+ compartment, required for the alternative MHC class I presentation, through autophagy. MHC class I–restricted CD8+, but not MHC class II–restricted CD4+, memory T cells failed to be generated in mice with CGD due to defective DC endosomal alkalinization and autophagy, which impeded sorting to the Rab14+ compartment. Note that the non-protective fungal antigen Mep1p colocalized rapidly with Rab9 and weakly with Lamp1, a finding suggesting that transporting antigens from late endosome to the TGN prevents adequate processing and presentation of antigens for CD4+ T cell activation.
## Comment in
* NADPH oxidase regulates efficacy of vaccination in aspergillosis. Deepe GS Jr. Deepe GS Jr. J Clin Invest. 2012 May;122(5):1608-11. doi: 10.1172/JCI63417. Epub 2012 Apr 23. J Clin Invest. 2012. PMID: 22523062 Free PMC article.
## Similar articles
* TLR3 essentially promotes protective class I-restricted memory CD8⁺ T-cell responses to Aspergillus fumigatus in hematopoietic transplanted patients. Carvalho A, De Luca A, Bozza S, Cunha C, D'Angelo C, Moretti S, Perruccio K, Iannitti RG, Fallarino F, Pierini A, Latgé JP, Velardi A, Aversa F, Romani L. Carvalho A, et al. Blood. 2012 Jan 26;119(4):967-77. doi: 10.1182/blood-2011-06-362582. Epub 2011 Dec 6. Blood. 2012. PMID: 22147891 * Robust T cell responses to aspergillosis in chronic granulomatous disease: implications for immunotherapy. Cruz CR, Lam S, Hanley PJ, Bear AS, Langston C, Cohen AJ, Liu H, Martinez CA, Krance RA, Heslop HE, Rooney CM, Hanson IC, Bollard CM. Cruz CR, et al. Clin Exp Immunol. 2013 Oct;174(1):89-96. doi: 10.1111/cei.12156. Clin Exp Immunol. 2013. PMID: 23763437 Free PMC article. * Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia. Rivera A, Van Epps HL, Hohl TM, Rizzuto G, Pamer EG. Rivera A, et al. Infect Immun. 2005 Nov;73(11):7170-9. doi: 10.1128/IAI.73.11.7170-7179.2005. Infect Immun. 2005. PMID: 16239511 Free PMC article. * MHC molecules and microbial antigen processing in phagosomes. Ramachandra L, Simmons D, Harding CV. Ramachandra L, et al. Curr Opin Immunol. 2009 Feb;21(1):98-104. doi: 10.1016/j.coi.2009.01.001. Epub 2009 Feb 11. Curr Opin Immunol. 2009. PMID: 19217269 Free PMC article. Review. * Vaccine-Induced CD8+ T Cell Responses in Children: A Review of Age-Specific Molecular Determinants Contributing to Antigen Cross-Presentation. Beijnen EMS, van Haren SD. Beijnen EMS, et al. Front Immunol. 2020 Dec 23;11:607977. doi: 10.3389/fimmu.2020.607977. eCollection 2020. Front Immunol. 2020. PMID: 33424857 Free PMC article. Review.
## Cited by
* PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice. Zhou J, Cheung AK, Tan Z, Wang H, Yu W, Du Y, Kang Y, Lu X, Liu L, Yuen KY, Chen Z. Zhou J, et al. J Clin Invest. 2013 Jun;123(6):2629-42. doi: 10.1172/JCI64704. Epub 2013 May 1. J Clin Invest. 2013. PMID: 23635778 Free PMC article. * Autophagy in dendritic cells. Ghislat G, Lawrence T. Ghislat G, et al. Cell Mol Immunol. 2018 Nov;15(11):944-952. doi: 10.1038/cmi.2018.2. Epub 2018 Mar 26. Cell Mol Immunol. 2018. PMID: 29578531 Free PMC article. Review. * Aspergillus fumigatus morphology and dynamic host interactions. van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latgé JP. van de Veerdonk FL, et al. Nat Rev Microbiol. 2017 Nov;15(11):661-674. doi: 10.1038/nrmicro.2017.90. Epub 2017 Sep 18. Nat Rev Microbiol. 2017. PMID: 28919635 Review. * IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, Gresnigt MS, Begun J, Plantinga TS, Joosten LA, van der Meer JW, Chamilos G, Netea MG, Xavier RJ, Dinarello CA, Romani L, van de Veerdonk FL. de Luca A, et al. Proc Natl Acad Sci U S A. 2014 Mar 4;111(9):3526-31. doi: 10.1073/pnas.1322831111. Epub 2014 Feb 18. Proc Natl Acad Sci U S A. 2014. PMID: 24550444 Free PMC article. * Noncanonical Fungal Autophagy Inhibits Inflammation in Response to IFN-γ via DAPK1. Oikonomou V, Moretti S, Renga G, Galosi C, Borghi M, Pariano M, Puccetti M, Palmerini CA, Amico L, Carotti A, Prezioso L, Spolzino A, Finocchi A, Rossi P, Velardi A, Aversa F, Napolioni V, Romani L. Oikonomou V, et al. Cell Host Microbe. 2016 Dec 14;20(6):744-757. doi: 10.1016/j.chom.2016.10.012. Epub 2016 Nov 23. Cell Host Microbe. 2016. PMID: 27889463 Free PMC article.
## References
1. 1. Aimanianda V, et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature. 2009;460(7259):1117–1121. doi: 10.1038/nature08264. - DOI - PubMed 2. 1. Felgentreff K, et al. Clinical and immunological manifestations of patients with atypical severe combined immunodeficiency. Clin Immunol. 2011;141(1):73–82. - PubMed 3. 1. Chaudhary N, Staab JF, Marr KA. Healthy human T-Cell Responses to Aspergillus fumigatus antigens. . PLoS One. 2010;5(2):e9036. doi: 10.1371/journal.pone.0009036. - DOI - PMC - PubMed 4. 1. Hebart H, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. . Blood. 2002;100(13):4521–4528. doi: 10.1182/blood-2002-01-0265. - DOI - PubMed 5. 1. Perruccio K, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. - PMC - PubMed
## Publication types
## MeSH terms
## Substances
## LinkOut - more resources
* ### Full Text Sources * American Society for Clinical Investigation * Europe PubMed Central * Ovid Technologies, Inc. * PubMed Central * ### Other Literature Sources * H1 Connect - Access expert opinions and insights on biomedical research. * The Lens - Patent Citations Database * ### Medical * MedlinePlus Health Information * ### Molecular Biology Databases * Mouse Genome Informatics (MGI) * ### Research Materials * NCI CPTC Antibody Characterization Program