Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury - PubMed (original) (raw)
Adora2b signaling on bone marrow derived cells dampens myocardial ischemia-reperfusion injury
Michael Koeppen et al. Anesthesiology. 2012 Jun.
Abstract
Background: Cardiac ischemia-reperfusion (I-R) injury represents a major cause of cardiac tissue injury. Adenosine signaling dampens inflammation during cardiac I-R. The authors investigated the role of the adenosine A2b-receptor (Adora2b) on inflammatory cells during cardiac I-R.
Methods: To study Adora2b signaling on inflammatory cells, the authors transplanted wild-type (WT) bone marrow (BM) into Adora2b(-/-) mice or Adora2b(-/-) BM into WT mice. To study the role of polymorphonuclear leukocytes (PMNs), neutrophil-depleted WT mice were treated with an Adora2b agonist. After treatments, mice were exposed to 60 min of myocardial ischemia and 120 min of reperfusion. Infarct sizes and troponin I concentrations were determined by triphenyltetrazolium chloride staining and enzyme-linked immunosorbent assay, respectively.
Results: Transplantation of WT BM into Adora2b(-/-) mice decreased infarct sizes by 19 ± 4% and troponin I by 87.5 ± 25.3 ng/ml (mean ± SD, n = 6). Transplantation of Adora2b(-/-) BM into WT mice increased infarct sizes by 20 ± 3% and troponin I concentrations by 69.7 ± 17.9 ng/ml (mean ± SD, n = 6). Studies on the reperfused myocardium revealed PMNs as the dominant cell type. PMN depletion or Adora2b agonist treatment reduced infarct sizes by 30 ± 11% or 26 ± 13% (mean ± SD, n = 4); however, the combination of both did not produce additional cardioprotection. Cytokine profiling showed significantly higher cardiac tumor necrosis factor α concentrations in Adora2b(-/-) compared with WT mice (39.3 ± 5.3 vs. 7.5 ± 1.0 pg/mg protein, mean ± SD, n = 4). Pharmacologic studies on human-activated PMNs revealed an Adora2b-dependent tumor necrosis factor α release.
Conclusion: Adora2b signaling on BM-derived cells such as PMNs represents an endogenous cardioprotective mechanism during cardiac I-R. The authors' findings suggest that Adora2b agonist treatment during cardiac I-R reduces tumor necrosis factor α release of PMNs, thereby dampening tissue injury.
Figures
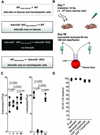
Figure 1. Adora2b in IR injury
(A) Overview of the 4 different experiment protocols used in this study. Abbreviations: **A/T**=Anesthesia/Thoracotomy; **BMTx**=Bone marrow transplantation; +/− **BAY**= with or without BAY 60–6583; **GR**-1= anti- (polymorphonuclear leukocyte) PMN antibody; **H&E**= hematoxylin and eosin stain; **IS**=Infarct size; **cTnI**=cardiac Troponin-I; **Cytokines**=Multiplex ELISA for cytokines. (B) Immunohistochemistry for the adenosine receptor A2b (Adora2b) revealed complete lack of Adora2b staining in _Adora2b_−/− animals while wild-type (WT) animals exhibit strong Adora2b protein expression in the heart and lung. (C) The absence of the Adora2b led to infarct sizes of 64±4%. Infarct sizes are presented as percentage of the area at risk (AAR). Serum Troponin-I levels were measured by ELISA. _Adora2b_−/− mice had Troponin-I values of 110±12 ng/ml, whereas WT had Troponin-I values of 36±4 ng/ml. (D) Two and three dimensional structure of BAY 60–6583, a highly specific Adora2b agonist used in this study. (E) WT mice were treated with BAY 60–6583 upon reperfusion and the infarct sizes and Troponin-I values were measured. BAY 60–6583 treatment reduced infarct sizes from 54 ± 5 to 28 ± 8%. Troponin-I values in the serum were reduced from 35 ± 5 to 8 ± 2 ng/ml by Adora2b agonist treatment; mean ±SD; n = 5 per group
Figure 2. Generation of Adora2b bone marrow chimeric mice
(A) Overview of the different bone-marrow-chimeric mice exposed to in situ myocardial ischemia (fig. 3). (B) Wild-type (WT) or adenosine receptor A2b minus (_Adora2b_−/−) mice were irradiated with 12 Gy to eliminate all bone-marrow derived cells. Next, 107 cells isolated from the WT or _Adora2b_−/− bone marrow were injected into mice after irradiation as indicated in (A). After 56 days these mice were submitted to an in vivo model of 60 min myocardial ischemia, followed by 120 min of reperfusion. (C) WT mice were treated with 12 gray (Gy) of irradiation to ablate all bone-marrow derived cells, and then reconstituted with 107 cells isolated from a donor animal bone marrow. Erythrocyte count as well as a Leucocyte count was performed to verify that the bone-marrow ablation was successful. Erythrocyte cell count did not change over time. Leucocytes linage was absent on day 3 (0.1 ± 0.1 × 106 cells/µl; n = 4) and day 4 (0.1 ± 0.1 × 106 cells/µl; n = 4) after irradiation treatment. Leucocytes count was at the same level as preirradiation value at day 56 after bone-marrow transplantation (day -1: 10.1 ± 0.5 × 106 cells/µl; day 56: 9.3 ± 0.5 × 106 cells/µl; mean ± SD; n = 4 per group). (D) WT mice underwent irradiation with 12 Gy to ablate all bone marrow derived cells. Then, irradiated WT mice received bone marrow from donor animal carrying the CD45.1 alloantigen on all bone marrow derived cells to reconstitute the white blood cell linage. Fluorescence-activated cell sorting analysis revealed that between 94 ± 0.6% (CD8+ T lymphocytes) and 98 ± 0.5% (B cell linage) of bone marrow derived cells carried the CD45.1 alloantigen ; mean ± SD; n = 5 per group.
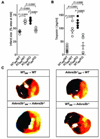
Figure 3. Infarct sizes and Troponin levels in Adora2b bone marrow–chimeric mice
(A)–(C) Bone marrow (BM) chimeric mice for the Adora2b were subjected to 60 min of myocardial ischemia and 120 min of reperfusion. (A) Infarct sizes are presented as percentage of the area at risk (AAR). The absence of the Adenosine receptor A2b (Adora2b) on bone marrow derived cells led to infarct sizes of 60±4% [_Adora2b_−/− _(KO)_BM → WT (wild-type)] and 63% ± 4% [_Adora2b_−/− _(KO)_BM → _Adora2b_−/−]. Adora2b presence on bone-marrow derived cells led to infarct sizes of 42 ± 2% (WTBM → WT) and 48 ± 1% [WTBM → _Adora2b_−/− _(KO)_]. (B) Measurement of serum Troponin-I levels by ELISA. Adora2b absence on bone-marrow derived cells increased Troponin-I values to 122 ± 8 ng/ml [_Adora2b_−/− _(KO)_BM → WT] and 92 ± 11 ng/ml [_Adora2b_−/− _(KO)_BM → Adora2b_−/−(KO)]. Adora2b presence on bone marrow derived cells reduced Troponin-I values to 22 ± 4 ng/ml (WTBM → WT) and 34 ± 13 ng/ml (WTBM → Adora2b_−/−(KO)). (C) Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Representative images of infarct sizes from the experiments in (A, B) are displayed; mean ± SD; n = 5 per group.
Figure 4. Ischemia-reperfusion associated histological changes
(A) Hematoxylin and eosin stain staining of the myocardial tissue after 60 min of ischemia and 15-min reperfusion. The cardiac structure appears normal with a few inflammatory cells (arrow) adhering to the endothelia. (B) Hematoxylin and eosin staining of the myocardium after 60 min of ischemia followed by 120 min of reperfusion. Multiple inflammatory cells adhering to or passing the endothelial cell layer (arrows; original magnification 400×). (C) Immunohistochemistry for Myeloperoxidase of myocardial tissue after 60 min of ischemia and 120 min of reperfusion. Multiple Myeloperoxidase positive cells adhere to the endothelial cell layer or transmigrate to the perivascular space (arrows; original magnification 200×). (D) Immunohistochemistry for F4–80 surface protein (expressed on monocytes/macrophages) after 60 min of ischemia by 120 min of reperfusion. Single F4–80+ cell adheres to the endothelial cell layer (arrow). (E) Immunohistochemistry for CD3 (T-cell-marker) after 60 min of ischemia and 120 min of reperfusion. Sporadic CD3+ cells attach to the endothelial cell layer (arrow). (F) Immunohistochemistry for Ly6G (polymorphonuclear leukozyte (PMN) marker) after 60 min of ischemia and 120 min of reperfusion. Multiple PMNs adhere to the endothelial cell layer and infiltrate the myocardium (arrows, original magnification 200×); shown are representative images from one experiment out of four mice.
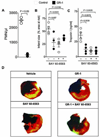
Figure 5. Adora2b signaling in PMN depleted mice
(A) Wild-type mice were treated with a monoclonal antibody directed against the granulocyte receptor 1 (GR-1) exclusively expressed on polymorphonuclear leukocytes (PMNs) 24 h before the experiment. GR-1 treatment decreased PMNs in peripheral blood from 2,500 ± 150 PMNs/µl (control) to 150 ± 75 PMNs (mean ± SD, n = 4 per group). (B) Infarct sizes are presented as percentage of the area at risk (AAR). The pre-treatment with a PMN-depleting antibody alone led to Infarct sizes of 28 ± 8%. Additional administration of BAY 60–6583 during reperfusion in the presence of PMNs led to infarct sizes of 24 ± 5%, and to 36 ± 2% in the absence of PMNs. (C) Measurement of serum Troponin-I levels by ELISA. Treatment with BAY 60–6583 during reperfusion resulted in Troponin I values to 10±4 ng/ml. GR-1-anitbody treatment resulted Troponin I values to 12 ± 6 ng/ml. PMNs-depletion and subsequent BAY 60–6583 administration in the reperfusion resulted in Troponin I values of 9 ± 4 ng/ml. (D) Infarct sizes were measured by double staining with Evan’s blue and triphenyltetrazolium chloride. Infarct sizes are expressed as the percent of the AAR that underwent infarction. Representative images of infarct sizes from the experiments in (B, C) are displayed; mean ± SD; n = 5 per group.
Figure 6. Cytokine profiling during ischemia reperfusion
(A–D) Wild-type (WT) or _Adora2b_−/− mice underwent 60 min ischemia followed by 120 min of reperfusion. The area at risk was harvested, homogenized and probed for various cytokines using a muliplex ELISA. Values were normalized to the total protein content of the tissue samples. (A) To quantify polymorphonuclear leukocytes (PMN) sequestration into the myocardium, an ELISA for Myeloperoxidase (MPO) was performed (WT: 1.4 ± 0.2 ng/mg Protein; _Adora2b_−/−: 1.5 ± 0.1 ng/mg protein; not significant). (B, C) In order to characterize the inflammatory response in WT and _Adora2b_−/−, Interferon-γ (Inf-γ), Interleukin -1β (IL-1β), Interleukin -2 (IL-2), Interleukin -4 (IL-4), Interleukin -5 (IL-5), Keratinocyte-derived Cytokine (KC), Interleukin - 10 (IL-10) and Interleukin -12 (IL-12) tissue levels were measured; Note: no significant changes were found whereas Interleukin-2 and Interleukin-10 were negative in both groups (data not shown). (D) Tumor necrosis factor-alpha (TNFα) content of the area at risk in WT and _Adora2b_−/− (WT: 7.5 ± 0.5 pg/mg protein; _Adora2b_−/−: 39.3 pg/mg protein); mean ± SD; n = 4 per group.
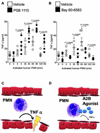
Figure 7. Adora2b signaling in human PMNs
(A, B) Human polymorphonuclear leukocytes (PMNs) were isolated from healthy individuals and activated using formyl-Met-Leu-Phe (fMLP 10 nM). Cells were either treated with a specific Adora2b antagonist (PSB 1115, 100 µM, a) or an Adora2b agonist (BAY 60–6583,1µM, b) or vehicle. Tumor necrosis factor-alpha (TNFα) release into the supernatant was measured by ELISA. Vehicle treatment of PMNs significantly increased TNFα with a maximal response after 30 and 60 min following activation (22.88 ± 4.0 pg/ml and 31.4 ± 5.4 pg/ml, respectively). (A) Pretreatment with an Adora2b antagonist enhances TNFα secretion significantly (24.8 ± 4.8, 42.3 ± 3.2, 48.6 ± 4.0 and 28 ± 4.6 pg/ml at 5, 30, 60, or 120 min, respectively). (B) Pretreatment with an Adora2b agonist significantly dampens TNFα release (7.89 ± 1.3, 10.70 ± 2.6, 15.9 ± 2.5 and 5.5 ± 0.9 pg/ml at 5, 30, 60, and 120 min, respectively; n = 5 per group). (C, D) Proposed role and potential therapeutic intervention: Cardiac ischemia reperfusion injury leads to PMN adhesion to the endothelial cells in the area at risk. (C) PMNs release TNFα, which further damages the myocardial tissue and enhances reperfusion injury. (D) Pharmacological treatment upon reperfusion using an Adora2b agonist activates A2B adenosine receptors on PMNs, which inhibits TNFα release and thereby protects the myocardium from further damage.
Similar articles
- Attenuating myocardial ischemia by targeting A2B adenosine receptors.
Eltzschig HK, Bonney SK, Eckle T. Eltzschig HK, et al. Trends Mol Med. 2013 Jun;19(6):345-54. doi: 10.1016/j.molmed.2013.02.005. Epub 2013 Mar 26. Trends Mol Med. 2013. PMID: 23540714 Free PMC article. Review. - Differential Tissue-Specific Function of Adora2b in Cardioprotection.
Seo SW, Koeppen M, Bonney S, Gobel M, Thayer M, Harter PN, Ravid K, Eltzschig HK, Mittelbronn M, Walker L, Eckle T. Seo SW, et al. J Immunol. 2015 Aug 15;195(4):1732-43. doi: 10.4049/jimmunol.1402288. Epub 2015 Jul 1. J Immunol. 2015. PMID: 26136425 Free PMC article. - Adora2b adenosine receptor signaling protects during acute kidney injury via inhibition of neutrophil-dependent TNF-α release.
Grenz A, Kim JH, Bauerle JD, Tak E, Eltzschig HK, Clambey ET. Grenz A, et al. J Immunol. 2012 Nov 1;189(9):4566-73. doi: 10.4049/jimmunol.1201651. Epub 2012 Oct 1. J Immunol. 2012. PMID: 23028059 Free PMC article. Retracted. - Signaling through hepatocellular A2B adenosine receptors dampens ischemia and reperfusion injury of the liver.
Zimmerman MA, Grenz A, Tak E, Kaplan M, Ridyard D, Brodsky KS, Mandell MS, Kam I, Eltzschig HK. Zimmerman MA, et al. Proc Natl Acad Sci U S A. 2013 Jul 16;110(29):12012-7. doi: 10.1073/pnas.1221733110. Epub 2013 Jun 28. Proc Natl Acad Sci U S A. 2013. PMID: 23812746 Free PMC article. Retracted. - The Hypoxia-Adenosine Link during Myocardial Ischemia-Reperfusion Injury.
Ruan W, Ma X, Bang IH, Liang Y, Muehlschlegel JD, Tsai KL, Mills TW, Yuan X, Eltzschig HK. Ruan W, et al. Biomedicines. 2022 Aug 10;10(8):1939. doi: 10.3390/biomedicines10081939. Biomedicines. 2022. PMID: 36009485 Free PMC article. Review.
Cited by
- CD73 Rather Than CD39 Is Mainly Involved in Controlling Purinergic Signaling in Calcified Aortic Valve Disease.
Kudryavtsev I, Serebriakova M, Zhiduleva E, Murtazalieva P, Titov V, Malashicheva A, Shishkova A, Semenova D, Irtyuga O, Isakov D, Mitrofanova L, Moiseeva O, Golovkin A. Kudryavtsev I, et al. Front Genet. 2019 Jul 25;10:604. doi: 10.3389/fgene.2019.00604. eCollection 2019. Front Genet. 2019. PMID: 31402927 Free PMC article. - ADORA2b Signaling in Cardioprotection.
Gile J, Eckle T. Gile J, et al. J Nat Sci. 2016;2(10):e222. J Nat Sci. 2016. PMID: 27747290 Free PMC article. - Attenuating myocardial ischemia by targeting A2B adenosine receptors.
Eltzschig HK, Bonney SK, Eckle T. Eltzschig HK, et al. Trends Mol Med. 2013 Jun;19(6):345-54. doi: 10.1016/j.molmed.2013.02.005. Epub 2013 Mar 26. Trends Mol Med. 2013. PMID: 23540714 Free PMC article. Review. - Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis.
Aherne CM, Saeedi B, Collins CB, Masterson JC, McNamee EN, Perrenoud L, Rapp CR, Curtis VF, Bayless A, Fletcher A, Glover LE, Evans CM, Jedlicka P, Furuta GT, de Zoeten EF, Colgan SP, Eltzschig HK. Aherne CM, et al. Mucosal Immunol. 2015 Nov;8(6):1324-38. doi: 10.1038/mi.2015.22. Epub 2015 Apr 8. Mucosal Immunol. 2015. PMID: 25850656 Free PMC article. - The Many Faces of the A2b Adenosine Receptor in Cardiovascular and Metabolic Diseases.
Eisenstein A, Patterson S, Ravid K. Eisenstein A, et al. J Cell Physiol. 2015 Dec;230(12):2891-7. doi: 10.1002/jcp.25043. J Cell Physiol. 2015. PMID: 25975415 Free PMC article. Review.
References
- Hausenloy DJ, Yellon DM. Time to take myocardial reperfusion injury seriously. N Engl J Med. 2008;359:518–520. - PubMed
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. - PubMed
- Jennewein C, Paulus P, Zacharowski K. Linking inflammation and coagulation: Novel drug targets to treat organ ischemia. Curr Opin Anaesthesiol. 2011;24:375–380. - PubMed
- Peart JN, Headrick JP. Adenosinergic cardioprotection: Multiple receptors, multiple pathways. Pharmacol Ther. 2007;114:208–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 HL102267-03/HL/NHLBI NIH HHS/United States
- K08 HL102267-01/HL/NHLBI NIH HHS/United States
- 1K08HL102267-01/HL/NHLBI NIH HHS/United States
- K08 HL102267/HL/NHLBI NIH HHS/United States
- K08 HL102267-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources