Loss of maternal CTCF is associated with peri-implantation lethality of Ctcf null embryos - PubMed (original) (raw)
Loss of maternal CTCF is associated with peri-implantation lethality of Ctcf null embryos
James M Moore et al. PLoS One. 2012.
Abstract
CTCF is a highly conserved, multifunctional zinc finger protein involved in critical aspects of gene regulation including transcription regulation, chromatin insulation, genomic imprinting, X-chromosome inactivation, and higher order chromatin organization. Such multifunctional properties of CTCF suggest an essential role in development. Indeed, a previous report on maternal depletion of CTCF suggested that CTCF is essential for pre-implantation development. To distinguish between the effects of maternal and zygotic expression of CTCF, we studied pre-implantation development in mice harboring a complete loss of function Ctcf knockout allele. Although we demonstrated that homozygous deletion of Ctcf is early embryonically lethal, in contrast to previous observations, we showed that the Ctcf nullizygous embryos developed up to the blastocyst stage (E3.5) followed by peri-implantation lethality (E4.5-E5.5). Moreover, one-cell stage Ctcf nullizygous embryos cultured ex vivo developed to the 16-32 cell stage with no obvious abnormalities. Using a single embryo assay that allowed both genotype and mRNA expression analyses of the same embryo, we demonstrated that pre-implantation development of the Ctcf nullizygous embryos was associated with the retention of the maternal wild type Ctcf mRNA. Loss of this stable maternal transcript was temporally associated with loss of CTCF protein expression, apoptosis of the developing embryo, and failure to further develop an inner cell mass and trophoectoderm ex vivo. This indicates that CTCF expression is critical to early embryogenesis and loss of its expression rapidly leads to apoptosis at a very early developmental stage. This is the first study documenting the presence of the stable maternal Ctcf transcript in the blastocyst stage embryos. Furthermore, in the presence of maternal CTCF, zygotic CTCF expression does not seem to be required for pre-implantation development.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
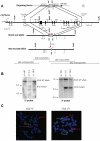
Figure 1. Ctcf maps to mouse chromosome 8.
The Jackson Laboratory interspecific backcross panels (BSS and BSB) were utilized to map the Ctcf locus to mouse chromosome 8. The loci are listed in order with the most proximal at the top. The black boxes represent the C57BL6/JEi allele and the white boxes the SPRET/Ei allele. The number of animals with each haplotype is given at the bottom of each column of boxes. The percent recombination (R) between adjacent loci is given to the right of the lower figure together with the standard error (SE) for each R.
Figure 2. Generation of Ctcf knockout mice.
(A) A schematic diagram of the mouse Ctcf locus and the targeting vector derived from the wild type Ctcf allele as described in Materials and Methods are shown. E1–E12 denote Ctcf exons 1 through 12. Restriction enzyme sites shown on map are as follows: E denotes _Eco_RI, X denotes _Xba_I, and S denotes _Spe_I. The locations of both the 5-prime and 3-prime Ctcf genomic probes for the Southern blot analysis are indicated. Red arrows show the locations of genotyping primers. A schematic diagram of genomic digest with _Eco_RV of the Ctcf knock-out and wild type alleles is shown below. (B) A Southern blot analysis of _Eco_RV digested genomic DNA utilizing the 5-prime and 3-prime Ctcf genomic probes distinguishes the wild type (14 kb or 25 kb) from the mutated (8 kb or 13 kb) Ctcf alleles. (C) FISH analysis of chromosome spreads from lymphocyte cultures derived from Ctcf heterozygous (+/−) and wild type (+/+) mice. The probe for Ctcf, a lambda phage clone 19.1 containing a 17 kb Ctcf genomic DNA insert that includes all coding Ctcf exons that were deleted in the Ctcf knockout allele (Figure 2A), was visualized with fluorescein (green fluorescence). The probe for chromosome 8 identification, a BAC clone (MB11301, Research Genetics), was visualized with Cy3 (red fluorescence). Examples of single metaphase cells from the Ctcf (+/−) and Ctcf (+/+) mice after hybridization to the Ctcf and chromosome 8 probes are shown. Both homologues of chromosome 8 are labeled with a red and a green signal in the wild type mouse, whereas absence of green signals on one homologue of chromosome 8 confirmed the presence of a deletion of Ctcf in the Ctcf (+/−) mouse.
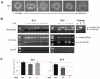
Figure 3. Ctcf (−/−) embryos retain maternal Ctcf mRNA and develop to the blastocyst stage.
(A) E0.5 one-cell stage embryos were isolated from Ctcf (+/−) heterozygous intercrosses and cultured ex vivo as detailed in Materials and Methods. (B) A single embryo assay shows genotyping and RT-PCR analysis of DNA and RNA simultaneously isolated from the same freshly isolated E3.5 and E4.5 pre-implantation stage embryos. The top panel shows a representative multiplex PCR-based genotyping analysis of genomic DNA from these embryos, which distinguishes wild type and mutated Ctcf alleles. The next panel shows multiplex RT-PCR analysis of mRNA from the same pre-implantation embryos for the presence of Ctcf and Gapdh transcripts. The bottom panel shows ‘no RT’ controls for the same samples. The displayed results are representative of over 60 pre-implantation embryos that were analyzed from intercrosses of Ctcf (+/−) heterozygous mice. _Hae_III digested ϕX DNA was utilized as DNA molecular weight markers in these gels. (C) Real time RT-PCR analysis of Ctcf expression was performed on genotyped E3.5 and E4.5 embryos from Ctcf (+/−) heterozygous intercrosses. Ctcf RNA levels were normalized to Gapdh expression. The results represent the mean of 3–6 independent embryos of the indicated genotype. Each embryo was assayed in triplicate, data represents mean +/− standard error.
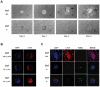
Figure 4. Ctcf nullizygous embryos fail to develop beyond the E3.5 stage and undergo apoptosis.
(A) Ex vivo outgrowth of E3.5 embryos. Blastocysts (E3.5) were isolated from Ctcf heterozygous (+/−) intercrosses and cultured ex vivo for the indicated number of days. At the end of the culture period the embryos were harvested and PCR genotyping and RT-PCR performed. The cultured Ctcf (+/+) and (+/−) embryos outgrew to form an inner cell mass (ICM) and trophoectoderm (TE) while little proliferation was noted in the cultured Ctcf (−/−) embryos (40×). (B) E3.5 blastocyst stage embryos isolated from the indicated crosses were subjected to DAPI staining and anti-CTCF immunochemistry (40×). All (16/16) embryos analyzed from the Ctcf (+/−) heterozygous intercrosses were positive for CTCF protein expression, displaying staining patterns similar to the two representative embryos shown here. The expected Mendelian ratio of genotypes in such crosses indicates that the chance that none of these 16 embryos were Ctcf (−/−) is less than 0.05 (i.e. (3/4)16≅0.01). (C) Day two outgrowths of E3.5 embryos of the indicated genotype. Blastocysts (E3.5) were cultured ex vivo for two days and then subjected to DAPI staining, anti-CTCF immunohistochemistry and TUNEL assay (40×). Ctcf (+/+) and (+/−) embryos outgrew and expressed CTCF, while cultured Ctcf (−/−) embryos failed to outgrow and exhibited loss of CTCF protein expression and increased apoptosis. The observations displayed here involve one Ctcf (+/+) and two Ctcf (−/−) embryos cultured ex vivo and are representative of over 60 embryos that were analyzed from Ctcf (+/−) heterozygous intercrosses.
Similar articles
- Maternal depletion of CTCF reveals multiple functions during oocyte and preimplantation embryo development.
Wan LB, Pan H, Hannenhalli S, Cheng Y, Ma J, Fedoriw A, Lobanenkov V, Latham KE, Schultz RM, Bartolomei MS. Wan LB, et al. Development. 2008 Aug;135(16):2729-38. doi: 10.1242/dev.024539. Epub 2008 Jul 9. Development. 2008. PMID: 18614575 Free PMC article. - Establishment of 3D chromatin structure after fertilization and the metabolic switch at the morula-to-blastocyst transition require CTCF.
Andreu MJ, Alvarez-Franco A, Portela M, Gimenez-Llorente D, Cuadrado A, Badia-Careaga C, Tiana M, Losada A, Manzanares M. Andreu MJ, et al. Cell Rep. 2022 Oct 18;41(3):111501. doi: 10.1016/j.celrep.2022.111501. Cell Rep. 2022. PMID: 36260992 - Deletion of beta 1 integrins in mice results in inner cell mass failure and peri-implantation lethality.
Stephens LE, Sutherland AE, Klimanskaya IV, Andrieux A, Meneses J, Pedersen RA, Damsky CH. Stephens LE, et al. Genes Dev. 1995 Aug 1;9(15):1883-95. doi: 10.1101/gad.9.15.1883. Genes Dev. 1995. PMID: 7544312 - The role of CCCTC-binding factor (CTCF) in genomic imprinting, development, and reproduction.
Franco MM, Prickett AR, Oakey RJ. Franco MM, et al. Biol Reprod. 2014 Nov;91(5):125. doi: 10.1095/biolreprod.114.122945. Epub 2014 Oct 8. Biol Reprod. 2014. PMID: 25297545 Review. - Test-tube embryos - mouse and human development in vitro to blastocyst stage and beyond.
Govindasamy N, Duethorn B, Oezgueldez HO, Kim YS, Bedzhov I. Govindasamy N, et al. Int J Dev Biol. 2019;63(3-4-5):203-215. doi: 10.1387/ijdb.180379ib. Int J Dev Biol. 2019. PMID: 31058297 Review.
Cited by
- When Dad's Stress Gets under Kid's Skin-Impacts of Stress on Germline Cargo and Embryonic Development.
Kretschmer M, Fischer V, Gapp K. Kretschmer M, et al. Biomolecules. 2023 Dec 6;13(12):1750. doi: 10.3390/biom13121750. Biomolecules. 2023. PMID: 38136621 Free PMC article. Review. - The chromatin insulator CTCF and the emergence of metazoan diversity.
Heger P, Marin B, Bartkuhn M, Schierenberg E, Wiehe T. Heger P, et al. Proc Natl Acad Sci U S A. 2012 Oct 23;109(43):17507-12. doi: 10.1073/pnas.1111941109. Epub 2012 Oct 8. Proc Natl Acad Sci U S A. 2012. PMID: 23045651 Free PMC article. - Detailed molecular and epigenetic characterization of the pig IPEC-J2 and chicken SL-29 cell lines.
de Vos J, Crooijmans RPMA, Derks MFL, Kloet SL, Dibbits B, Groenen MAM, Madsen O. de Vos J, et al. iScience. 2023 Feb 20;26(3):106252. doi: 10.1016/j.isci.2023.106252. eCollection 2023 Mar 17. iScience. 2023. PMID: 36936794 Free PMC article. - Organizational principles of 3D genome architecture.
Rowley MJ, Corces VG. Rowley MJ, et al. Nat Rev Genet. 2018 Dec;19(12):789-800. doi: 10.1038/s41576-018-0060-8. Nat Rev Genet. 2018. PMID: 30367165 Free PMC article. Review. - Cross-species meta-analysis of transcriptome changes during the morula-to-blastocyst transition: metabolic and physiological changes take center stage.
Schall PZ, Latham KE. Schall PZ, et al. Am J Physiol Cell Physiol. 2021 Dec 1;321(6):C913-C931. doi: 10.1152/ajpcell.00318.2021. Epub 2021 Oct 20. Am J Physiol Cell Physiol. 2021. PMID: 34669511 Free PMC article.
References
- Lobanenkov VV, Nicolas RH, Adler VV, Paterson H, Klenova EM, et al. A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene. 1990;5:1743–1753. - PubMed
- Filippova GN. Genetics and epigenetics of the multifunctional protein CTCF. Curr Top Dev Biol. 2008;80:337–360. - PubMed
- Ohlsson R, Renkawitz R, Lobanenkov V. CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet. 2001;17:520–527. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- GM 46883/GM/NIGMS NIH HHS/United States
- R01 CA068360/CA/NCI NIH HHS/United States
- CA68360/CA/NCI NIH HHS/United States
- R01 GM046883/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases