Angiotensin II facilitates breast cancer cell migration and metastasis - PubMed (original) (raw)
doi: 10.1371/journal.pone.0035667. Epub 2012 Apr 20.
Mohamed Abdelkarim, Patricia Dillenburg-Pilla, Anny-Claude Luissint, Anne di-Tommaso, Frédérique Deshayes, Carmen Lucia S Pontes, Angie Molina, Nicolas Cagnard, Franck Letourneur, Marina Morel, Rosana I Reis, Dulce E Casarini, Benoit Terris, Pierre-Olivier Couraud, Claudio M Costa-Neto, Mélanie Di Benedetto, Clara Nahmias
Affiliations
- PMID: 22536420
- PMCID: PMC3334979
- DOI: 10.1371/journal.pone.0035667
Angiotensin II facilitates breast cancer cell migration and metastasis
Sylvie Rodrigues-Ferreira et al. PLoS One. 2012.
Abstract
Breast cancer metastasis is a leading cause of death by malignancy in women worldwide. Efforts are being made to further characterize the rate-limiting steps of cancer metastasis, i.e. extravasation of circulating tumor cells and colonization of secondary organs. In this study, we investigated whether angiotensin II, a major vasoactive peptide both produced locally and released in the bloodstream, may trigger activating signals that contribute to cancer cell extravasation and metastasis. We used an experimental in vivo model of cancer metastasis in which bioluminescent breast tumor cells (D3H2LN) were injected intra-cardiacally into nude mice in order to recapitulate the late and essential steps of metastatic dissemination. Real-time intravital imaging studies revealed that angiotensin II accelerates the formation of metastatic foci at secondary sites. Pre-treatment of cancer cells with the peptide increases the number of mice with metastases, as well as the number and size of metastases per mouse. In vitro, angiotensin II contributes to each sequential step of cancer metastasis by promoting cancer cell adhesion to endothelial cells, trans-endothelial migration and tumor cell migration across extracellular matrix. At the molecular level, a total of 102 genes differentially expressed following angiotensin II pre-treatment were identified by comparative DNA microarray. Angiotensin II regulates two groups of connected genes related to its precursor angiotensinogen. Among those, up-regulated MMP2/MMP9 and ICAM1 stand at the crossroad of a network of genes involved in cell adhesion, migration and invasion. Our data suggest that targeting angiotensin II production or action may represent a valuable therapeutic option to prevent metastatic progression of invasive breast tumors.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
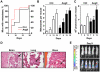
Figure 1. AngII increases the time-course, incidence and number of metastases in an experimental model in vivo.
(A). Percentage of mice showing at least one detectable metastasis over time after intracardiac injection of D3H2LN cells treated with AngII (red dotted line, n = 14) or vehicle (black line, n = 15). (B). Number of metastases per mouse at indicated days. Results are mean +/− SEM of 15 control (white bar) and 14 AngII-treated (black bar) groups. (C). Number of photons/s per mouse at indicated days. Results are expressed as in B. (D). Histological analysis of metastases developing at the brain (left panel), the lung (middle panel) and the bone (right panel), obtained from 3 µm sections of formalin-fixed, paraffin-embedded tissue blocks stained with hematoxylin/eosin. Arrows indicate tumor cells. Magnification, 200x. (E). Representative pictures of 5 mice taken at day 9 after injection of control cells (upper panel) or AngII-treated cells (lower panel). * p<0.05, ** p<0.01.
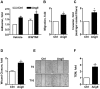
Figure 2. AngII increases breast cancer cell adhesion and migration.
(A). MDA-MB-231 breast cancer cell adhesion to HCMEC/D3 endothelial cells monolayer following exposure of cancer cells to AngII (100 nM) for 24 hrs. Results are means +/− SEM of 7 independent experiments performed in quadruplicate, and expressed as fold increase of untreated cells (control, Ctrl). *p<0.05. (B, C). Boyden chamber assays of tumor cell migration across 8 µm-pore filters either non coated (B) or coated with matrigel to mimic cell invasion (C). Results are means +/− SEM of 3 separate experiments performed in triplicate, and expressed as fold increase of control. *p<0.05. (D, E). Wound healing assay. Results are from 2 independent experiments performed in quintuplicate, and expressed as fold increase of wound closure at time 16 hrs (T16) compared to control (vehicle-treated cells). *p<0.05. (E). Representative pictures of wounds from control and AngII-treated cells (100 nM, 24 hrs) at T0 and T16. Magnification, 100x. (F). Trans-endothelial migration. Results are mean +/− SEM of 3 independent experiments performed in triplicate, and expressed as fold increase of control. *p<0.05.
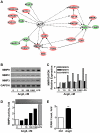
Figure 3. AngII transcriptionally regulates a panel of connected genes.
(A). Gene networks differentially regulated by AngII. Up- and down-regulated genes related to angiotensinogen (AGT) are indicated in red and green, respectively. Filled lines indicate direct interactions, filled and dashed arrows indicate direct and indirect regulations, respectively. Note two groups of connected genes centered around MAPK1 and MMP2/9, respectively. (B). RT-PCR analysis of MMP9, MMP2 and MMP3 mRNA expression in MDA-MB-231 cells treated for 24 hrs with increasing doses of AngII as indicated, or LPS (Lipopolysaccharide, 100 ng/ml) as a positive control. GAPDH amplification was used as internal control. Shown is one out of 3 to 5 independent experiments performed in duplicate. (C). Quantification (Image J software) of PCR amplification of MMP9, MMP2 and MMP3 relative to GAPDH and normalized to expression levels in cells treated with 1 nM AngII. (D). Gelatin-based zymography analysis of MMP9 activity in conditioned medium of cells treated as in B. Shown is one representative out of 3 independent experiments (Upper panel). Quantification (ImageJ software) of results normalized to the quantity of proteins in cell lysate and expressed relative to control (lower panel). (E). FACS analysis of ICAM-1 expression at the plasma membrane of MDA-MB-231 cells treated with AngII (100 nM) or vehicle for 24 hrs. Results are means +/− SEM of 3 independent experiments and expressed as fold-increase of the control. **p<0.01.
Similar articles
- Role of the blood-brain barrier in the formation of brain metastases.
Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. Wilhelm I, et al. Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. Int J Mol Sci. 2013. PMID: 23344048 Free PMC article. Review. - Invading basement membrane matrix is sufficient for MDA-MB-231 breast cancer cells to develop a stable in vivo metastatic phenotype.
Abdelkarim M, Vintonenko N, Starzec A, Robles A, Aubert J, Martin ML, Mourah S, Podgorniak MP, Rodrigues-Ferreira S, Nahmias C, Couraud PO, Doliger C, Sainte-Catherine O, Peyri N, Chen L, Mariau J, Etienne M, Perret GY, Crepin M, Poyet JL, Khatib AM, Di Benedetto M. Abdelkarim M, et al. PLoS One. 2011;6(8):e23334. doi: 10.1371/journal.pone.0023334. Epub 2011 Aug 15. PLoS One. 2011. PMID: 21858074 Free PMC article. - Profilin-1 downregulation has contrasting effects on early vs late steps of breast cancer metastasis.
Ding Z, Joy M, Bhargava R, Gunsaulus M, Lakshman N, Miron-Mendoza M, Petroll M, Condeelis J, Wells A, Roy P. Ding Z, et al. Oncogene. 2014 Apr 17;33(16):2065-74. doi: 10.1038/onc.2013.166. Epub 2013 May 20. Oncogene. 2014. PMID: 23686314 Free PMC article. - Plantamajoside, a potential anti-tumor herbal medicine inhibits breast cancer growth and pulmonary metastasis by decreasing the activity of matrix metalloproteinase-9 and -2.
Pei S, Yang X, Wang H, Zhang H, Zhou B, Zhang D, Lin D. Pei S, et al. BMC Cancer. 2015 Dec 16;15:965. doi: 10.1186/s12885-015-1960-z. BMC Cancer. 2015. PMID: 26674531 Free PMC article. - A methodological approach to unravel organ-specific breast cancer metastasis.
Nola S, Sin S, Bonin F, Lidereau R, Driouch K. Nola S, et al. J Mammary Gland Biol Neoplasia. 2012 Jun;17(2):135-45. doi: 10.1007/s10911-012-9256-2. Epub 2012 May 25. J Mammary Gland Biol Neoplasia. 2012. PMID: 22628182 Review.
Cited by
- ATIP3 deficiency facilitates intracellular accumulation of paclitaxel to reduce cancer cell migration and lymph node metastasis in breast cancer patients.
Rodrigues-Ferreira S, Nehlig A, Kacem M, Nahmias C. Rodrigues-Ferreira S, et al. Sci Rep. 2020 Aug 6;10(1):13217. doi: 10.1038/s41598-020-70142-7. Sci Rep. 2020. PMID: 32764625 Free PMC article. - Astrocytes enhance the invasion potential of glioblastoma stem-like cells.
Rath BH, Fair JM, Jamal M, Camphausen K, Tofilon PJ. Rath BH, et al. PLoS One. 2013;8(1):e54752. doi: 10.1371/journal.pone.0054752. Epub 2013 Jan 22. PLoS One. 2013. PMID: 23349962 Free PMC article. - Renin-Angiotensin System in Lung Tumor and Microenvironment Interactions.
Catarata MJ, Ribeiro R, Oliveira MJ, Robalo Cordeiro C, Medeiros R. Catarata MJ, et al. Cancers (Basel). 2020 Jun 3;12(6):1457. doi: 10.3390/cancers12061457. Cancers (Basel). 2020. PMID: 32503281 Free PMC article. Review. - Role of the blood-brain barrier in the formation of brain metastases.
Wilhelm I, Molnár J, Fazakas C, Haskó J, Krizbai IA. Wilhelm I, et al. Int J Mol Sci. 2013 Jan 11;14(1):1383-411. doi: 10.3390/ijms14011383. Int J Mol Sci. 2013. PMID: 23344048 Free PMC article. Review. - 20(S)-Protopanaxadiol Inhibits Angiotensin II-Induced Epithelial- Mesenchymal Transition by Downregulating SIRT1.
Wang Y, Xu H, Fu W, Lu Z, Guo M, Wu X, Sun M, Liu Y, Yu X, Sui D. Wang Y, et al. Front Pharmacol. 2019 May 7;10:475. doi: 10.3389/fphar.2019.00475. eCollection 2019. Front Pharmacol. 2019. PMID: 31133857 Free PMC article.
References
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. - PubMed
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous