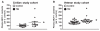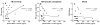Elevated plasma MCP-1 concentration following traumatic brain injury as a potential "predisposition" factor associated with an increased risk for subsequent development of Alzheimer's disease - PubMed (original) (raw)
Elevated plasma MCP-1 concentration following traumatic brain injury as a potential "predisposition" factor associated with an increased risk for subsequent development of Alzheimer's disease
Lap Ho et al. J Alzheimers Dis. 2012.
Abstract
We explored whether changes in the expression profile of peripheral blood plasma proteins may provide a clinical, readily accessible "window" into the brain, reflecting molecular alterations following traumatic brain injury (TBI) that might contribute to TBI complications. We recruited fourteen TBI and ten control civilian participants for the study, and also analyzed banked plasma specimens from 20 veterans with TBI and 20 control cases. Using antibody arrays and ELISA assays, we explored differentially-regulated protein species in the plasma of TBI compared to healthy controls from the two independent cohorts. We found three protein biomarker species, monocyte chemotactic protein-1 (MCP-1), insulin-like growth factor-binding protein-3, and epidermal growth factor receptor, that are differentially regulated in plasma specimens of the TBI cases. A three-biomarker panel using all three proteins provides the best potential criterion for separating TBI and control cases. Plasma MCP-1 contents are correlated with the severity of TBI and the index of compromised axonal fiber integrity in the frontal cortex. Based on these findings, we evaluated postmortem brain specimens from 7 mild cognitive impairment (MCI) and 7 neurologically normal cases. We found elevated MCP-1 expression in the frontal cortex of MCI cases that are at high risk for developing Alzheimer's disease. Our findings suggest that additional application of the three-biomarker panel to current diagnostic criteria may lead to improved TBI detection and more sensitive outcome measures for clinical trials. Induction of MCP-1 in response to TBI might be a potential predisposing factor that may increase the risk for development of Alzheimer's disease.
Figures
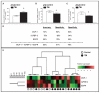
Fig. 1
Candidate plasma biomarker contents provide a sensitive and specific criterion for distinguishing TBI from control cases. Relative expression of 120 known signaling proteins in plasma specimens from a subset of 12 TBI and 8 control cases from the civilian study cohort (Table 1) were assessed by antibody array platforms (RayBio Cytokine Antibody Array 2000; Raybiotech Inc.). The student _t_-test was used to test the significance of the protein expression differences between TBI and control cases. (A–C) Outcomes of the antibody array study led to the identification of three candidate TBI biomarkers: MCP-1, IGFBP-3, and EGFR. Bar graphs represent mean ± SD of plasma biomarker contents for MCP-1 (A), IGFBP-3 (B), and EGFR (C) among TBI and control cases. *2-tailed student _t_-test, p < 0.05, TBI compared to the control group. (D, E) Relative plasma biomarker contents assessed by antibody arrays were tested by unsupervised clustering analysis using the UPGMA algorithm with cosine correlation as the similarity metric. (D) Summary table of analysis results using individual MCP-1, IGFBP-3, and EGFR or using a combination of all three-protein species (the “three-protein” model). Accuracy represents the percentage of all 20 TBI and normal healthy controls in the antibody array profile analysis study that were correctly diagnosed by the test, calculated as the number of correctly identified TBI and normal healthy controls divided by the total number of patients in this study. Sensitivity (true positive [TP]/[TP + false negative (FN)]) is the probability that a patient who was predicted to have TBI actually has it, whereas the specificity (true negative [TN]/[false positive (FP) + TN]) measures the probability that a patient predicted not to have TBI will, in fact, not have it. (E) A heat map graphically depicting the efficacy of using a three biomarker panel to distinguish TBI and control cases by unsupervised clustering analysis.
Fig. 2
Validation of plasma MCP-1 content as a clinically accessible TBI biomarker in two demographically distinct TBI study cohorts. An independent, quantitative ELISA assay was used to assess plasma MCP-1 contents in TBI and control cases from a civilian and a military veteran study cohort. (A) Plasma MCP-1 contents in TBI (n = 14) and control (n = 10) cases from the civilian study cohort (Table 1). (B) Plasma MCP-1 contents among veteran TBI (n = 20) and control (n = 20) cases (Table 2). Scatter graphs represent values for individual cases and group mean values ± SEM of plasma biomarker contents, expressed as % of controls. *p < 0.05 by student _t_-test, TBI compared to the control group.
Fig. 3
Correlations of plasma MCP-1 content with self-reported indices of TBI complications. Correlation analyses were conducted using self-reported indices of TBI (assessed by BISQ [37, 38]) that were available for the civilian study cohort of 14 TBI and 10 control cases (Table 1). Correlations of plasma MCP-1 contents (pg/ml) with (A) TBI severity (p = 0.029), (B) a summation of 25 cognitive symptoms that are sensitive and specific to TBI [38] (p = 0.757), and (C) self-assessments of mood (p = 0.871).
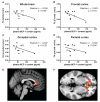
Fig. 4
Plasma MCP-1 contents are inversely correlated with brain FA measures. Correlational analyses were conducted using DTI information that was available for female (7 TBI and 5 control) cases from the civilian study cohort (Table 1). FA, a measure of white matter integrity [72], was calculated using the FSL comprehensive library of analysis tools for brain imaging data (
). Correlation of plasma MCP-1 contents (pg/ml) with whole brain FA (A), frontal cortex FA (B), occipital cortex FA (C), and parietal cortex FA (D). Representative sagittal (E) and transverse (F) sections of the brain illustrating significant correlations of FA with plasma MCP-1 contents (indicated by red-yellow) in frontal white matter and the genu of the corpus callosum.
Fig. 5
Elevated MCP-1 mRNA in frontal cortex (BM9) brain specimens of cases characterized by mild cognitive impairment. (A) Contents of MCP-1 mRNA were assessed by qPCR and normalized to brain contents of human TATA-binding protein mRNA, used as an internal control. Bar graphs represent mean (± SEM) normalized MCP-1 mRNA contents in BM9 of CDR 0 cases (n = 6), expressed as % of MCP-1 mRNA contents in control cases (n = 6). *Student _t_-test, p < 0.05. B–D) Correlation analysis of BM9 MCP-1 mRNA contents with (B) postmortem interval, (C) age of death, and (D) neuritic plaque density (per mm2). There was no significant correlation between brain MCP-1 mRNA contents and postmortem interval (p = 0.534), age of death (p = 0.201), or neuritic plaque density (p = 0.105).
Fig. 6
Proposed mechanisms by which TBI may increase risk for Alzheimer’s disease and other neurological disorders. Schematic represents an overview of a proposed model by which TBI exposure may mechanistically increase subsequent risk for Alzheimer’s disease and other neurological disorders. Accordingly, TBI resulting from either mechanical injuries or blast injuries, which are predominant, respectively, in the civilian or the Veteran population, induces biological responses that lead to aberrant biochemical, structural and/or functional changes in the brain. For example, long-term induction of MCP-1 following TBI may contribute to demyelination processes that, ultimately, may reduce resilience of the brain to subsequent neurodegenerative insults.
Similar articles
- Decreased level of olfactory receptors in blood cells following traumatic brain injury and potential association with tauopathy.
Zhao W, Ho L, Varghese M, Yemul S, Dams-O'Connor K, Gordon W, Knable L, Freire D, Haroutunian V, Pasinetti GM. Zhao W, et al. J Alzheimers Dis. 2013;34(2):417-429. doi: 10.3233/JAD-121894. J Alzheimers Dis. 2013. PMID: 23241557 Free PMC article. - Plasma MCP-1 and Cognitive Decline in Patients with Alzheimer's Disease and Mild Cognitive Impairment: A Two-year Follow-up Study.
Lee WJ, Liao YC, Wang YF, Lin IF, Wang SJ, Fuh JL. Lee WJ, et al. Sci Rep. 2018 Jan 19;8(1):1280. doi: 10.1038/s41598-018-19807-y. Sci Rep. 2018. PMID: 29352259 Free PMC article. - Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease.
Galimberti D, Fenoglio C, Lovati C, Venturelli E, Guidi I, Corrà B, Scalabrini D, Clerici F, Mariani C, Bresolin N, Scarpini E. Galimberti D, et al. Neurobiol Aging. 2006 Dec;27(12):1763-8. doi: 10.1016/j.neurobiolaging.2005.10.007. Epub 2005 Nov 22. Neurobiol Aging. 2006. PMID: 16307829 - Traumatic brain injury and Alzheimer's disease: a review.
Van Den Heuvel C, Thornton E, Vink R. Van Den Heuvel C, et al. Prog Brain Res. 2007;161:303-16. doi: 10.1016/S0079-6123(06)61021-2. Prog Brain Res. 2007. PMID: 17618986 Review. - CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis.
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, Hölttä M, Rosén C, Olsson C, Strobel G, Wu E, Dakin K, Petzold M, Blennow K, Zetterberg H. Olsson B, et al. Lancet Neurol. 2016 Jun;15(7):673-684. doi: 10.1016/S1474-4422(16)00070-3. Epub 2016 Apr 8. Lancet Neurol. 2016. PMID: 27068280 Review.
Cited by
- Neuroimaging of traumatic brain injury in military personnel: An overview.
Bhattrai A, Irimia A, Van Horn JD. Bhattrai A, et al. J Clin Neurosci. 2019 Dec;70:1-10. doi: 10.1016/j.jocn.2019.07.001. Epub 2019 Jul 19. J Clin Neurosci. 2019. PMID: 31331746 Free PMC article. Review. - Adipokines: a link between obesity and dementia?
Kiliaan AJ, Arnoldussen IA, Gustafson DR. Kiliaan AJ, et al. Lancet Neurol. 2014 Sep;13(9):913-23. doi: 10.1016/S1474-4422(14)70085-7. Lancet Neurol. 2014. PMID: 25142458 Free PMC article. Review. - Diffusion Tensor Imaging of TBI: Potentials and Challenges.
Douglas DB, Iv M, Douglas PK, Anderson A, Vos SB, Bammer R, Zeineh M, Wintermark M. Douglas DB, et al. Top Magn Reson Imaging. 2015 Oct;24(5):241-51. doi: 10.1097/RMR.0000000000000062. Top Magn Reson Imaging. 2015. PMID: 26502306 Free PMC article. Review. - Anti-inflammatory interleukin 1 receptor antagonist concentration in plasma correlates with blood-brain barrier integrity in the primary lesion area in traumatic brain injury patients.
To XV, Donnelly P, Maclachlan L, Mahady K, Apellaniz EM, Cumming P, Winter C, Nasrallah F. To XV, et al. Brain Behav Immun Health. 2023 Jun 22;31:100653. doi: 10.1016/j.bbih.2023.100653. eCollection 2023 Aug. Brain Behav Immun Health. 2023. PMID: 37415924 Free PMC article. - Testing a Multivariate Proteomic Panel for Traumatic Brain Injury Biomarker Discovery: A TRACK-TBI Pilot Study.
Huie JR, Diaz-Arrastia R, Yue JK, Sorani MD, Puccio AM, Okonkwo DO, Manley GT, Ferguson AR; TRACK-TBI Investigators. Huie JR, et al. J Neurotrauma. 2019 Jan 1;36(1):100-110. doi: 10.1089/neu.2017.5449. Epub 2018 Sep 27. J Neurotrauma. 2019. PMID: 30084741 Free PMC article.
References
- Centers for Disease Control and Prevention . Traumatic brain injury in the United States: A report to congress. 1999. http://www.cdc.gov/ncipc/pub-res/tbi_congress/TBI_in_the_US.PDF.
- McDowell S, Whyte J, D’Esposito M. Working memory impairments in traumatic brain injury: Evidence from a dual-task paradigm. Neuropsychologia. 1997;35:1341–1353. - PubMed
- Schretlen DJ, Shapiro AM. A quantitative review of the effects of traumatic brain injury on cognitive functioning. Int Rev Psychiatry. 2003;15:341–349. - PubMed
- Kou Z, Wu Z, Tong KA, Holshouser B, Benson RR, Hu J, Haacke EM. The role of advanced MR imaging findings as biomarkers of traumatic brain injury. J Head Trauma Rehabil. 2010;25:267–282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1RR029887/RR/NCRR NIH HHS/United States
- AG05138/AG/NIA NIH HHS/United States
- I01 RX000521/RX/RRD VA/United States
- P50NS062684/NS/NINDS NIH HHS/United States
- P50AG05136/AG/NIA NIH HHS/United States
- P50 AG005138/AG/NIA NIH HHS/United States
- I01 RX001612/RX/RRD VA/United States
- UL1 RR029887/RR/NCRR NIH HHS/United States
- PPG AG02219/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
- P01 AG002219/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous