Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy - PubMed (original) (raw)
Tumor-targeted TNFα stabilizes tumor vessels and enhances active immunotherapy
Anna Johansson et al. Proc Natl Acad Sci U S A. 2012.
Abstract
Solid tumors are intrinsically resistant to immune rejection. Abnormal tumor vasculature can act as a barrier for immune cell migration into tumors. We tested whether targeting IFNγ and/or TNFα into pancreatic neuroendocrine tumors can alleviate immune suppression. We found that intratumoral IFNγ causes rapid vessel loss, which does not support anti-tumor immunity. In contrast, low-dose TNFα enhances T-cell infiltration and overall survival, an effect that is exclusively mediated by CD8(+) effector cells. Intriguingly, lymphocyte influx does not correlate with increased vessel leakiness. Instead, low-dose TNFα stabilizes the vascular network and improves vessel perfusion. Inflammatory vessel remodeling is, at least in part, mediated by tumor-resident macrophages that are reprogrammed to secrete immune and angiogenic modulators. Moreover, inflammatory vessel remodeling with low-dose TNFα substantially improves antitumor vaccination or adoptive T-cell therapy. Thus, low-dose TNFα promotes both vessel remodeling and antitumor immune responses and acts as a potent adjuvant for active immunotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
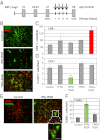
IFNγ and TNFα have distinct effects in the tumor microenvironment. (A) Schematic representation of a short-term treatment regimen in RIP1-Tag5 mice. Arrows indicate four i.v. injections of compounds. Tumors were analyzed at 29 wk. (B) Costaining of control (untreated), IFNγ–RGR and TNFα-RGR treated tumors with specific antibodies: CD8+ T cells, red; CD31+ blood vessels, green. Representative pictures after biweekly i.v. injections of 2 μg of IFNγ–RGR or TNFα-RGR for 2 wk are shown. (Original magnification: 20×) (Scale bar: 100 μm.) (C) Quantification of tumor-infiltrating CD8+ T cells (mean CD8+ T cells per field ± SE, n = 3–9, *P < 0.01 compared with all other groups). (D) Quantification of CD31-positive blood vessels (mean % of CD31-covered area/field ± SE, n = 4–6, *P ≤ 0.01 compared with all other treatment groups). (E) Costaining of CD31-positive blood vessels (red) with TUNEL+, apoptotic cells (green) in IFNγ–RGR treated tumors. (Original magnification: 10×) (Scale bar: 200 μm.) Inset shows clustering of apoptotic cells around a vessel. (Original magnification: 40×) (Scale bar: 50 μm.) (F) Quantification of apoptotic cells in different treatment groups (mean TUNEL+ cells per field ± SE, n = 3–7, *P = 0.02 compared with control and TNFα-RGR treated groups).
Fig. 2.
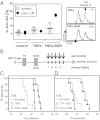
Long-term survival under TNFα-RGR monotherapy is CD8+ T-cell dependent. (A) Untreated or TNFα/TNFα-RGR treated RIP1-Tag5/F1 mice were assessed for in vivo CTL activity against the Tag-specific peptide IV after 2 wk of treatment. (Left) Combined data for spleen cells (n = 5) and tumor-draining pancreatic lymph nodes (LN; n = 3–5, LNs were pooled in each of two independent experiments). (Right) Representative histograms of percent specific kill of CFSEhigh LN cells from two treatment groups. (B) Long-term treatment scheme: RIP1-Tag5 mice were treated at the age of 22–23 wk with biweekly i.v. injections and survival monitored. Percent survival of RIP1-Tag5 mice treated with 2 μg of TNFα or TNFα-RGR (P = 0.002, TNFα-RGR compared with TNFα; P = 0.001, TNFα-RGR compared with untreated controls (n = 5–7) (C), and 2 μg of TNFα-RGR in the presence (αCD8) and absence (IgG) of CD8+ T-cell depleting antibodies (P = 0.0002, TNFα-RGR plus depletion compared with TNFα-RGR with control IgG, n = 8) (D).
Fig. 3.
Intratumoral TNFα-RGR enhances efficacy of anticancer immunotherapy. (A) RIP1-Tag5 mice were treated with vaccine alone, 2 μg of TNFα-RGR alone or in combination with anti-Tag vaccine (P = 0.007 single versus combination treatment, n = 8–12). (B) RIP1-Tag5 mice were treated every second week with adoptive transfers (ad T) of preactivated CD4+ and CD8+ Tag-specific T cells alone or in combination with 2 μg of TNFα-RGR, survival was monitored up to 45 wk (P < 0.0001, n = 8–10). (C) Percentage of TagTCR8 T cells in pancreatic lymph nodes (panc LN) or tumors was tracked by FACS analysis for 21 d after adoptive transfer in untreated (Left) or TNFα-RGR treated mice (Right), n = 3.
Fig. 4.
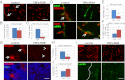
Tumor-targeted TNFα stabilizes vessels and enhances vascular functionality. (A) Representative pictures of CD31-positive vessels in control (untreated) RIP1-Tag5 tumors and after 2 wk of treatment with 2 μg of TNFα-RGR. Arrows point at large vessels. (Original magnification: 20×.) (Scale bar: 100 μm.) (B) Quantification of mean vessel length in control (Ctrl) and treatment groups (T-R, TNFα-RGR) (P = 0.01). (C) Quantification of percentage of large vessels (size: 150–200 μm) (P = 0.003). (D Upper) CD31+ vessels (green) and coverage with PDGFRβ+ pericytes (red). Arrow points at a pericyte-covered area in controls. (Lower) CD31+ vessels (red) and association of αSMA+ perivascular cells (green). Arrow points at close vascular lining in TNFα-RGR treated tumors. (Original magnification: 40×.) (Scale bar: 50 μm.) (E) Ratio of PDGFRβ-positive pericytes to CD31-positive endothelial cells (P = 0.003). (F) Percent αSMA+ covered endothelial cells (P = 0.02). (G) Vascular permeability assessed by injection of 70-kDa Texas-red labeled dextran followed by saline perfusion. (Upper) Dextran signals in tumors. Arrows point at areas of dextran extravasation. Arrowheads point at residual dextran associated with vessels. (Lower) Dextran/dapi double staining. (Original magnification: 20×.) (Scale bar: 100 μm.) (H) Quantification of percentage of dextran in tumors as readout for vascular leakiness (P = 0.02). (I) CD31-positive vessels (Upper) in relation to i.v. injected FITC-lectin (Lower). Dashed line indicates perfused and nonperfused tumor areas. (Original magnification: 20×.) (Scale bar: 100 μm.) (J) Ratio lectin-positive vessels to CD31-positive vessels (P = 0.03, n = 3–8 for all groups).
Fig. 5.
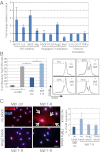
Tumor macrophages are activated and reprogrammed to express immunostimulatory factors and angiogenic modulators. (A) Quantitative PCR analysis of isolated CD68+ macrophages from TNFα-RGR treated tumors, expressed as fold change relative to CD68+ from control (untreated) RIP1-Tag5 tumors (n = 3). (B Left) Quantitative analysis of TagTCR8 cell proliferation, unstimulated (-) or stimulated with Tag-specific peptide/IL2 (+) in the presence of macrophages (MØ) isolated from untreated controls (ctrl) or tumors after 2 wk of treatment with 2 μg of TNFα-RGR (T-R) (P = 0.01). (Right) Representative histograms showing percent proliferation of CFSE-labeled T cells from all groups. (C Left) HUVEC were incubated with macrophages isolated from untreated (MØ ctrl) or TNFα-RGR treated tumors (MØ T-R) in the presence of Ang2 receptor (Tie2) (Ang2 block) or TNFα blocking antibodies (TNFα block). Arrows delineate VCAM positive, cellular HUVEC staining. (Original magnification: 40×.) (Scale bar: 50 μm.) (C Right) Quantification of percent VCAM-positive cells in relation to DAPI-positive cells (P = 0.08).
Similar articles
- Vascular-targeted TNFα and IFNγ inhibits orthotopic colorectal tumor growth.
Shen J, Li ZJ, Li LF, Lu L, Xiao ZG, Wu WK, Zhang L, Li MX, Hu W, Chan KM, Cho CH. Shen J, et al. J Transl Med. 2016 Jun 24;14(1):187. doi: 10.1186/s12967-016-0944-3. J Transl Med. 2016. PMID: 27342639 Free PMC article. - CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction.
Garbi N, Arnold B, Gordon S, Hämmerling GJ, Ganss R. Garbi N, et al. J Immunol. 2004 May 15;172(10):5861-9. doi: 10.4049/jimmunol.172.10.5861. J Immunol. 2004. PMID: 15128765 - IFNγ potentiates TNFα/TNFR1 signaling to induce FAT10 expression in macrophages.
Kandel-Kfir M, Garcia-Milan R, Gueta I, Lubitz I, Ben-Zvi I, Shaish A, Shir L, Harats D, Mahajan M, Canaan A, Kamari Y. Kandel-Kfir M, et al. Mol Immunol. 2020 Jan;117:101-109. doi: 10.1016/j.molimm.2019.11.004. Epub 2019 Nov 20. Mol Immunol. 2020. PMID: 31759325 - Vascular-targeted TNFα improves tumor blood vessel function and enhances antitumor immunity and chemotherapy in colorectal cancer.
Lu L, Li ZJ, Li LF, Wu WK, Shen J, Zhang L, Chan RL, Yu L, Liu YW, Ren SX, Chan KM, Cho CH. Lu L, et al. J Control Release. 2015 Jul 28;210:134-46. doi: 10.1016/j.jconrel.2015.05.282. Epub 2015 May 21. J Control Release. 2015. PMID: 26003042 - Tumor Vasculature Targeted TNFα Therapy: Reversion of Microenvironment Anergy and Enhancement of the Anti-tumor Efficiency.
Balza E, Carnemolla B, Orecchia P, Rubartelli A, Poggi A, Mortara L. Balza E, et al. Curr Med Chem. 2020;27(25):4233-4248. doi: 10.2174/0929867325666180904121118. Curr Med Chem. 2020. PMID: 30182839 Review.
Cited by
- Vascular-targeted TNFα and IFNγ inhibits orthotopic colorectal tumor growth.
Shen J, Li ZJ, Li LF, Lu L, Xiao ZG, Wu WK, Zhang L, Li MX, Hu W, Chan KM, Cho CH. Shen J, et al. J Transl Med. 2016 Jun 24;14(1):187. doi: 10.1186/s12967-016-0944-3. J Transl Med. 2016. PMID: 27342639 Free PMC article. - Vascular normalization as an emerging strategy to enhance cancer immunotherapy.
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK. Huang Y, et al. Cancer Res. 2013 May 15;73(10):2943-8. doi: 10.1158/0008-5472.CAN-12-4354. Epub 2013 Feb 25. Cancer Res. 2013. PMID: 23440426 Free PMC article. Review. - Homing to solid cancers: a vascular checkpoint in adoptive cell therapy using CAR T-cells.
Ager A, Watson HA, Wehenkel SC, Mohammed RN. Ager A, et al. Biochem Soc Trans. 2016 Apr 15;44(2):377-85. doi: 10.1042/BST20150254. Biochem Soc Trans. 2016. PMID: 27068943 Free PMC article. Review. - Microfluidic models for adoptive cell-mediated cancer immunotherapies.
Adriani G, Pavesi A, Tan AT, Bertoletti A, Thiery JP, Kamm RD. Adriani G, et al. Drug Discov Today. 2016 Sep;21(9):1472-1478. doi: 10.1016/j.drudis.2016.05.006. Epub 2016 May 13. Drug Discov Today. 2016. PMID: 27185084 Free PMC article. Review. - Immunotherapies targeting tumor vasculature: challenges and opportunities.
Dianat-Moghadam H, Nedaeinia R, Keshavarz M, Azizi M, Kazemi M, Salehi R. Dianat-Moghadam H, et al. Front Immunol. 2023 Sep 1;14:1226360. doi: 10.3389/fimmu.2023.1226360. eCollection 2023. Front Immunol. 2023. PMID: 37727791 Free PMC article. Review.
References
- Nelson D, Ganss R. Tumor growth or regression: Powered by inflammation. J Leukoc Biol. 2006;80:685–690. - PubMed
- Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–4681. - PubMed
- Garbi N, Arnold B, Gordon S, Hämmerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–5869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials