5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals - PubMed (original) (raw)
. 2012 May 2;32(18):6364-72.
doi: 10.1523/JNEUROSCI.5793-11.2012.
Chul-Kyu Park, Carlo Angioni, Dong Dong Zhang, Christian von Hehn, Enrique J Cobos, Nader Ghasemlou, Zhen-Zhong Xu, Vigneswara Kumaran, Ruirui Lu, Andrew Grant, Michael J M Fischer, Achim Schmidtko, Peter Reeh, Ru-Rong Ji, Clifford J Woolf, Gerd Geisslinger, Klaus Scholich, Christian Brenneis
Affiliations
- PMID: 22553041
- PMCID: PMC3359875
- DOI: 10.1523/JNEUROSCI.5793-11.2012
5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals
Marco Sisignano et al. J Neurosci. 2012.
Abstract
Epoxyeicosatrienoic acids (EETs) are cytochrome P450-epoxygenase-derived metabolites of arachidonic acid that act as endogenous signaling molecules in multiple biological systems. Here we have investigated the specific contribution of 5,6-EET to transient receptor potential (TRP) channel activation in nociceptor neurons and its consequence for nociceptive processing. We found that, during capsaicin-induced nociception, 5,6-EET levels increased in dorsal root ganglia (DRGs) and the dorsal spinal cord, and 5,6-EET is released from activated sensory neurons in vitro. 5,6-EET potently induced a calcium flux (100 nm) in cultured DRG neurons that was completely abolished when TRPA1 was deleted or inhibited. In spinal cord slices, 5,6-EET dose dependently enhanced the frequency, but not the amplitude, of spontaneous EPSCs (sEPSCs) in lamina II neurons that also responded to mustard oil (allyl isothiocyanate), indicating a presynaptic action. Furthermore, 5,6-EET-induced enhancement of sEPSC frequency was abolished in TRPA1-null mice, suggesting that 5,6-EET presynaptically facilitated spinal cord synaptic transmission by TRPA1. Finally, in vivo intrathecal injection of 5,6-EET caused mechanical allodynia in wild-type but not TRPA1-null mice. We conclude that 5,6-EET is synthesized on the acute activation of nociceptors and can produce mechanical hypersensitivity via TRPA1 at central afferent terminals in the spinal cord.
Figures
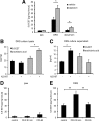
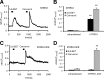
Figure 1.
5,6-EET concentrations in DRG tissue and release from sensory neurons upon activation. A, EET synthesis after nociceptive activation. 5,6-EET concentrations in the paw, L4–L6 DRGs, and the dorsal horn of L4–L6 spinal cords were measured 30 min after intraplantar injection of capsaicin (2 μg/25 μl) or vehicle. EET levels were determined from tissue extracts by LC-MS/MS. Shown is the average ± SEM form tissues of 10 animals per group. B, C, Levels of AA and 5,6-EET from cell lysates and supernatants of cultured DRG neurons. Neuron-enriched cultures from DRGs were incubated with A23187 (2 μ
m
) for 2 h. Then EETs and AA were extracted from cell lysates (B) or supernatants (C) and quantified by LC-MS/MS analysis. Data shown represent the average ± SEM from five culture dishes. D, E, Tissues from the plantar side of the paw (D), and L4–6 DRGs (E) were dissected 30 min or 6 h after intraplantar injection of 20 μl of CFA or vehicle. EET levels were determined by LC-MS/MS. Shown is the average ± SEM form tissues of six animals per group. *p ≤ 0.05, **p ≤ 0.01; Student's t test.
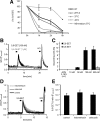
Figure 2.
5,6-EET induces calcium influx in sensory neurons. A, In vitro stability of 5,6-EET was tested under all buffer conditions (pH, temperature) used in this study. 5,6-EET (1 μ
m
) was dissolved in each buffer and was incubated for up to 6 h. 5,6-EET concentrations were measured from extracted buffers by LC-MS/MS. B, Stimulation of adult DRG neurons with 5,6-EET (100 n
m
, 10 s), but not its metabolite 5,6-DHET (1 μ
m
, 10 s), induced a transient and reversible calcium flux. Neurons were identified by responses to KCl (40 m
m
, 10 s). Shown is a representative trace. C, 5,6-EET dose dependently activates a maximal of 11% of DRG neurons. Cells were stimulated with different 5,6-EET concentrations or acetonitrile (ACN) as vehicle control (n = 5–6 experiments). D, Effects of COX inhibitors on 5,6-EET-mediated calcium flux in wild-type DRGs. Cultured DRG neurons were treated with 1 μ
m
indomethacin, 1 μ
m
celecoxib, or vehicle (0.1% DMSO, v/v) 1 h before stimulation with 5,6-EET (250 n
m
) and KCl (40 m
m
, 30 s each). Shown are representative traces. E, Statistical analysis of peak amplitudes from DRG neurons stimulated with 5,6-EET, as in D (n = 6 experiments each). NB, Neurobasal; RT, room temperature.
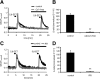
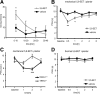
Figure 3.
5,6-EET induces calcium influx in DRG neurons by activation of TRP channels. A, 5,6-EET-induced calcium influx is blocked by Ca2+-free EGTA buffer. Ca2+-free EGTA buffer was washed in 5 min before a second 5,6-EET stimulation (100 n
m
, 10 s). Shown are representative traces of control (black) and Ca2+-free treated DRG neurons (gray). B, Average values of peak amplitudes normalized to the control (first 5,6-EET stimulation) peaks of traces from cells as stimulated in A (n = 5–6 experiments). C, 5,6-EET-induced calcium influx is blocked by RR. Five micrometers of RR were washed in for 5 min before DRG neurons were again stimulated with 5,6-EET (100 n
m
, 10 s). Shown are representative traces of control (black) and RR (5 μ
m
; gray) treated DRG neurons. D, Average values of peak amplitudes normalized to the control (first 5,6-EET stimulation) peaks of traces as in C (n = 6–7 experiments). **p < 0.01; Student's t test.
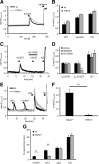
Figure 4.
5,6-EET-induced calcium influx in sensory neurons is mediated by TRPA1. A, 5,6-EET-induced calcium influx does not depend on TRPV4. 5,6-EET (300 n
m
, 10 s)-induced calcium fluxes in DRG neurons from wild-type and TRPV4−/− mice were compared. Shown is a representative trace. B, Statistical analysis of neurons as shown in A (n = 150–200 cells). C, Effect of the selective TRPA1-antagonist HC-030031 (20 μ
m
) on 5,6-EET (250 n
m
)-induced calcium flux. Shown is a representative trace. D, Statistical analysis of the effects of the TRPV1 (AMG9810, 1 μ
m
) and TRPA1 (HC-030031, 20 μ
m
) antagonists on 5,6-EET-induced peak amplitudes (n = 5–6 experiments). E, DRG neurons from TRPA1−/− mice (gray) respond less to AITC (100 μ
m
, 30 s) and not to 5,6-EET (250 n
m
, 30 s) but do respond similarly to capsaicin (250 n
m
, 30 s) and KCl (40 m
m
, 30 s). Shown are representative traces. F, Statistical analysis of the peak amplitudes from recordings of wild-type and TRPA1-deficient DRG neurons after 5,6-EET stimulation (250 n
m
). Shown is the average ± SEM from six to eight experiments. G, Percentage of responding cells stimulated as shown in E from wild-type and TRPA1-deficient DRG neurons. Shown is the average ± SEM from six to eight experiments. **p < 0.01; Student's t test. caps., Capsaicin; wt, wild-type.
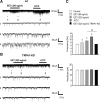
Figure 5.
Role of regulatory cysteine residues for 5,6-EET-induced TRPA1 activation. A, HEK-293 cells were transiently transfected with plasmids expressing (h)TRPA1 and stimulated with 5,6-EET (250 n
m
, 30 s) and carvacrol (250 μ
m
30 s). Shown is a representative trace. B, Statistical analysis of the peak amplitudes of recordings from cells stimulated as in A (n = 8 experiments). C, HEK-293 cells were transiently transfected with plasmids expressing the (h)TRPA1 3CK mutant and stimulated as in A. Shown is a representative trace. D, Statistical analysis of the peak amplitudes of recordings from cells as in C (n = 9 experiments). **p ≤ 0.01; Student's t test.
Figure 6.
Peripheral TRPA1 activation by 5,6-EET causes acute pain and mechanical allodynia. A, Spontaneous pain induced by 5,6-EET. After intraplantar injection of 20 μl of 5,6-EET (5 μ
m
) or vehicle (acetonitrile, 1.6%, v/v), the licking time was monitored (n = 8 animals per group). B, Mechanical allodynia after 5,6-EET injection. Dynamic plantar test after intraplantar injections of 20 μl of 5,6-EET (5 μ
m
) or vehicle (n = 8–9 animals per group). C, Comparison of mechanical allodynia in wild-type and TRPA1-deficient mice after intraplantar injection of 20 μl of 5,6-EET (5 μ
m
) or vehicle (n = 8 animals per group). D, 5,6-EET does not sensitize responses to a radiant heat stimulus. Hargreaves test after intraplantar injections of 20 μl of 5,6-EET (5 μ
m
) or vehicle (n = 10 animals per group). *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001; two-way repeated-measures ANOVA followed by Bonferroni's post-test. i.plantar, Intraplantar; PWL, paw withdrawal latency.
Figure 7.
5,6-EET enhances sEPSC frequency in lamina II neurons of spinal cord slices through TRPA1. A, B, sEPSC traces recorded in spinal cord slices of wild-type (A) and TRPA1-KO (B) mice. 1, Trace before EET treatment; 2, trace after EET treatment; 3, trace after AITC treatment (300 μ
m
). Note that EET- and AITC-induced sEPSC increases are abolished in TRPA1-KO mice. C, sEPSC frequency (top) and amplitude (bottom). Note that EET and AITC increase only the frequency but not the amplitude of sEPSCs. *p < 0.05, compared with pretreatment baseline (n = 5–10 cells). KO, Knock-out; wt, wild-type.
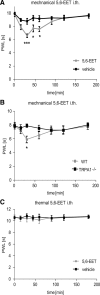
Figure 8.
Intrathecal injections of 5,6-EET cause mechanical allodynia through the activation of TRPA1. A, 5,6-EET reduces mechanical thresholds when injected intrathecally. Five microliters of 5,6-EET (10 μ
m
) or vehicle (DMSO, 3.2%, v/v) were injected intrathecally, followed by determination of the mechanical pain thresholds at time points 15, 30, 45, 60, 90, 120, and 180 min after injection using a dynamic plantar aesthesiometer. Shown is the average ± SEM paw withdrawal latency from eight animals per group. B, Comparison of mechanical thresholds in wild-type and TRPA1-deficient mice after intrathecal injections of 10 μl of 5,6-EET (10 μ
m
) using a dynamic plantar aesthesiometer. Shown is the average ± SEM from eight animals per group. C, Comparison of thermal thresholds in wild-type mice after intrathecal 5,6-EET injection (10 μ
m
) at the same time points shown in A using a Hargreaves apparatus. Shown is the average ± SEM from six animals per group. *p ≤ 0.05, ***p ≤ 0.001; two-way ANOVA followed by Bonferroni's post-test. i.th, Intrathecally; PWL, paw withdrawal latency; WT, wild-type.
Similar articles
- Soluble epoxide hydrolase limits mechanical hyperalgesia during inflammation.
Brenneis C, Sisignano M, Coste O, Altenrath K, Fischer MJ, Angioni C, Fleming I, Brandes RP, Reeh PW, Woolf CJ, Geisslinger G, Scholich K. Brenneis C, et al. Mol Pain. 2011 Oct 4;7:78. doi: 10.1186/1744-8069-7-78. Mol Pain. 2011. PMID: 21970373 Free PMC article. - MicroRNA let-7b enhances spinal cord nociceptive synaptic transmission and induces acute and persistent pain through neuronal and microglial signaling.
Chen O, Jiang C, Berta T, Powell Gray B, Furutani K, Sullenger BA, Ji RR. Chen O, et al. Pain. 2024 Aug 1;165(8):1824-1839. doi: 10.1097/j.pain.0000000000003206. Epub 2024 Mar 6. Pain. 2024. PMID: 38452223 - Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception.
Raisinghani M, Zhong L, Jeffry JA, Bishnoi M, Pabbidi RM, Pimentel F, Cao DS, Evans MS, Premkumar LS. Raisinghani M, et al. Am J Physiol Cell Physiol. 2011 Sep;301(3):C587-600. doi: 10.1152/ajpcell.00465.2010. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653898 Free PMC article. - Quantification and Potential Functions of Endogenous Agonists of Transient Receptor Potential Channels in Patients With Irritable Bowel Syndrome.
Cenac N, Bautzova T, Le Faouder P, Veldhuis NA, Poole DP, Rolland C, Bertrand J, Liedtke W, Dubourdeau M, Bertrand-Michel J, Zecchi L, Stanghellini V, Bunnett NW, Barbara G, Vergnolle N. Cenac N, et al. Gastroenterology. 2015 Aug;149(2):433-44.e7. doi: 10.1053/j.gastro.2015.04.011. Epub 2015 Apr 22. Gastroenterology. 2015. PMID: 25911511 - TRPA1 contributes to specific mechanically activated currents and sensory neuron mechanical hypersensitivity.
Brierley SM, Castro J, Harrington AM, Hughes PA, Page AJ, Rychkov GY, Blackshaw LA. Brierley SM, et al. J Physiol. 2011 Jul 15;589(Pt 14):3575-93. doi: 10.1113/jphysiol.2011.206789. Epub 2011 May 9. J Physiol. 2011. PMID: 21558163 Free PMC article.
Cited by
- Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain.
Sisignano M, Angioni C, Park CK, Meyer Dos Santos S, Jordan H, Kuzikov M, Liu D, Zinn S, Hohman SW, Schreiber Y, Zimmer B, Schmidt M, Lu R, Suo J, Zhang DD, Schäfer SM, Hofmann M, Yekkirala AS, de Bruin N, Parnham MJ, Woolf CJ, Ji RR, Scholich K, Geisslinger G. Sisignano M, et al. Proc Natl Acad Sci U S A. 2016 Nov 1;113(44):12544-12549. doi: 10.1073/pnas.1613246113. Epub 2016 Oct 17. Proc Natl Acad Sci U S A. 2016. PMID: 27791151 Free PMC article. - Terpenes and lipids of the endocannabinoid and transient-receptor-potential-channel biosignaling systems.
Janero DR, Makriyannis A. Janero DR, et al. ACS Chem Neurosci. 2014 Nov 19;5(11):1097-106. doi: 10.1021/cn5000875. Epub 2014 Jun 5. ACS Chem Neurosci. 2014. PMID: 24866555 Free PMC article. Review. - Omeprazole increases the efficacy of a soluble epoxide hydrolase inhibitor in a PGE₂ induced pain model.
Goswami SK, Inceoglu B, Yang J, Wan D, Kodani SD, da Silva CA, Morisseau C, Hammock BD. Goswami SK, et al. Toxicol Appl Pharmacol. 2015 Dec 15;289(3):419-27. doi: 10.1016/j.taap.2015.10.018. Epub 2015 Nov 10. Toxicol Appl Pharmacol. 2015. PMID: 26522832 Free PMC article. - TRPA1 as a drug target--promise and challenges.
Chen J, Hackos DH. Chen J, et al. Naunyn Schmiedebergs Arch Pharmacol. 2015 Apr;388(4):451-63. doi: 10.1007/s00210-015-1088-3. Epub 2015 Feb 3. Naunyn Schmiedebergs Arch Pharmacol. 2015. PMID: 25640188 Free PMC article. Review. - Gallic acid functions as a TRPA1 antagonist with relevant antinociceptive and antiedematogenic effects in mice.
Trevisan G, Rossato MF, Tonello R, Hoffmeister C, Klafke JZ, Rosa F, Pinheiro KV, Pinheiro FV, Boligon AA, Athayde ML, Ferreira J. Trevisan G, et al. Naunyn Schmiedebergs Arch Pharmacol. 2014 Jul;387(7):679-89. doi: 10.1007/s00210-014-0978-0. Epub 2014 Apr 11. Naunyn Schmiedebergs Arch Pharmacol. 2014. PMID: 24722818
References
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS039518/NS/NINDS NIH HHS/United States
- R37 NS039518/NS/NINDS NIH HHS/United States
- 2R37 NS039518-08/NS/NINDS NIH HHS/United States
- 1P01NS072040/NS/NINDS NIH HHS/United States
- P01 NS072040/NS/NINDS NIH HHS/United States
- R01 NS038253/NS/NINDS NIH HHS/United States
- BB/E527098/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases