Temporal dynamics of the human vaginal microbiota - PubMed (original) (raw)
. 2012 May 2;4(132):132ra52.
doi: 10.1126/scitranslmed.3003605.
Rebecca M Brotman, Guoyun Bai, Joyce Sakamoto, Ursel M E Schütte, Xue Zhong, Sara S K Koenig, Li Fu, Zhanshan Sam Ma, Xia Zhou, Zaid Abdo, Larry J Forney, Jacques Ravel
Affiliations
- PMID: 22553250
- PMCID: PMC3722878
- DOI: 10.1126/scitranslmed.3003605
Temporal dynamics of the human vaginal microbiota
Pawel Gajer et al. Sci Transl Med. 2012.
Abstract
Elucidating the factors that impinge on the stability of bacterial communities in the vagina may help in predicting the risk of diseases that affect women's health. Here, we describe the temporal dynamics of the composition of vaginal bacterial communities in 32 reproductive-age women over a 16-week period. The analysis revealed the dynamics of five major classes of bacterial communities and showed that some communities change markedly over short time periods, whereas others are relatively stable. Modeling community stability using new quantitative measures indicates that deviation from stability correlates with time in the menstrual cycle, bacterial community composition, and sexual activity. The women studied are healthy; thus, it appears that neither variation in community composition per se nor higher levels of observed diversity (co-dominance) are necessarily indicative of dysbiosis.
Conflict of interest statement
Competing interests. The authors declare that they have no competing interests.
Figures
Fig. 1
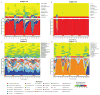
Dynamics of vaginal community state types in 32 women over 16 weeks. (A) Heatmap showing the proportions of community state types (I, II, III, IV-A and IV-B) observed within a woman over time (color key is indicated below panel C) that were used to generate the dendogram that depicts distances between proportions of the five community state types identified. (B) Color bar indicating community classes designated DA, LC, LG, DB, and LI and as defined by clusters of proportions of community state types within a woman over time. (C) Profiles of community state types for 32 women over 16 weeks. Each dot (white or black) represents one sample in the time series. The absence of a dot indicates missing samples. The community state types in the time series for each woman are color-coded according to the schema shown below panel C (see also fig. S1). (D) Box plot of normalized Jensen-Shannon distances between all pairs of community states within each subject. Community deviation from constancy is represented by the Jensen-Shannon Index (black bar, see Supplementary Online Materials), with higher values reflecting decreased constancy. (E) Box plot of average Nugent scores for each women over 16 weeks. Panels D and E, the whiskers represent the lowest and highest datum still within 1.5 interquartile range (IQR) of the lower and upper quartile. The middle 50% of the data is represented by the height of the box.
Fig. 2
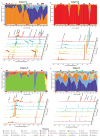
(A–D) Heatmaps (top) and interpolated bar plots (bottom) of phylotype relative abundance observed in four selected subjects over 16 weeks (heatmap color key is indicated in the lower right corner). Color codes for each phylotype represented in the interpolated bar plots are shown below the figure. See fig. S5 for heatmaps and interpolated bar plots for all subjects. Red dots below the interpolated bar graphs represent menstruation days.
Fig. 3
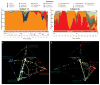
Temporal dynamics of vaginal bacterial communities in two women over 16 weeks. (A, C) Interpolated bar graph of phylotype relative abundance for subjects 13 (A) and 26 (C). Profiles of community state types in which Nugent scores have been superimposed (high Nugent score, 7–10, large open circles; intermediate Nugent score, 4–6, medium open circles; and low Nugent score, 1–3, small open circles) are shown below the interpolated bar graphs. Daily metadata is represented by the following: red balls, menstruation; black open square, douching; open red triangle, vaginal intercourse; open black diamond, oral sex; vertical black bar, digital penetration (insertion of finger(s) in the vagina); light blue closed circle, lubricant use. (B, D) Representation of vaginal community dynamics in 3D community space (3) for subjects 13 (B) and 26 (D). Communities dominated by species of Lactobacillus and representing community state types I (red), II (light blue), III (green), and V (purple) are shown at each of the four outer vertices of the tetrahedron, with community state type IV (yellow) at the inner vertex. The white line represents the succession of community states for each subject over time. Animations of these events are provided in the Supplementary Online Materials (movie S1 and movie S2).
Fig. 4
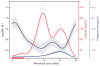
Modeling the dependence of the log of Jensen-Shannon divergence rate of change over the menstrual cycle. The length of menstrual cycles within and between women were normalized to 28 days (Supplementary Online Materials) with day 1 to 5 corresponding to menses (red bar). The red line shows concentrations of estradiol as a function of menstrual time (data from (21)) and the blue line shows concentrations of progesterone (data from (21)). The shaded areas around each curve show 95% point-wise confidence bands.
Fig. 5
Metabolomic analyses. 1H-NMR metabolome profiles for 4 representative subjects at selected time points throughout the 16-week study (A–D). An interpolated bar graph of phylotype relative abundance in each community is shown above the 1H-NMR profiles. The colored rectangles on top of the interpolated bar graph match the color of the 1H-NMR profiles and indicate the times of collection during the 16-week study. 1H-NMR spectra are spaced according to the matching time scale on the y-axis. Peaks for selected metabolites are labeled. Red arrow indicates lactic acid peaks, light blue arrow indicates succinate peak and green arrow indicates acetic acid peak. Red dots below the interpolated bar graphs represent menstruation days.
Comment in
- Complexities of the uniquely human vagina.
Witkin SS, Ledger WJ. Witkin SS, et al. Sci Transl Med. 2012 May 2;4(132):132fs11. doi: 10.1126/scitranslmed.3003944. Sci Transl Med. 2012. PMID: 22553249
Similar articles
- Effects of tampons and menses on the composition and diversity of vaginal microbial communities over time.
Hickey RJ, Abdo Z, Zhou X, Nemeth K, Hansmann M, Osborn TW 3rd, Wang F, Forney LJ. Hickey RJ, et al. BJOG. 2013 May;120(6):695-704; discussion 704-6. doi: 10.1111/1471-0528.12151. Epub 2013 Feb 11. BJOG. 2013. PMID: 23398859 Clinical Trial. - Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis.
Ling Z, Kong J, Liu F, Zhu H, Chen X, Wang Y, Li L, Nelson KE, Xia Y, Xiang C. Ling Z, et al. BMC Genomics. 2010 Sep 7;11:488. doi: 10.1186/1471-2164-11-488. BMC Genomics. 2010. PMID: 20819230 Free PMC article. - Amylases in the Human Vagina.
Nunn KL, Clair GC, Adkins JN, Engbrecht K, Fillmore T, Forney LJ. Nunn KL, et al. mSphere. 2020 Dec 9;5(6):e00943-20. doi: 10.1128/mSphere.00943-20. mSphere. 2020. PMID: 33298571 Free PMC article. - Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English. - The vaginal microbiome: new information about genital tract flora using molecular based techniques.
Lamont RF, Sobel JD, Akins RA, Hassan SS, Chaiworapongsa T, Kusanovic JP, Romero R. Lamont RF, et al. BJOG. 2011 Apr;118(5):533-49. doi: 10.1111/j.1471-0528.2010.02840.x. Epub 2011 Jan 20. BJOG. 2011. PMID: 21251190 Free PMC article. Review.
Cited by
- Vaginal microbiota in term pregnant women with differences in cervical ripeness revealed by 2bRAD-M.
Lu S, Wu Q, He W, Du X, Cui Q, Yang Y, Yin Z. Lu S, et al. BMC Microbiol. 2024 Nov 1;24(1):444. doi: 10.1186/s12866-024-03612-x. BMC Microbiol. 2024. PMID: 39487440 Free PMC article. - Female reproductive tract microbiota varies with MHC profile.
Leclaire S, Bandekar M, Rowe M, Ritari J, Jokiniemi A, Partanen J, Allinen P, Kuusipalo L, Kekäläinen J. Leclaire S, et al. Proc Biol Sci. 2024 Oct;291(2033):20241334. doi: 10.1098/rspb.2024.1334. Epub 2024 Oct 30. Proc Biol Sci. 2024. PMID: 39471862 - Antimicrobial Activity of Corynebacterium amycolatum ICIS 53 and Corynebacterium amycolatum ICIS 82 Against Urogenital Isolates of Multidrug-Resistant Staphylococcus aureus.
Gladysheva IV, Stroganova EA, Chertkov KL, Cherkasov SV. Gladysheva IV, et al. Curr Microbiol. 2024 Oct 24;81(12):426. doi: 10.1007/s00284-024-03936-x. Curr Microbiol. 2024. PMID: 39448409 - Metal availability shapes early life microbial ecology and community succession.
Soto Ocaña J, Friedman ES, Keenan O, Bayard NU, Ford E, Tanes C, Munneke MJ, Beavers WN, Skaar EP, Bittinger K, Zemel BS, Wu GD, Zackular JP. Soto Ocaña J, et al. mBio. 2024 Nov 13;15(11):e0153424. doi: 10.1128/mbio.01534-24. Epub 2024 Oct 23. mBio. 2024. PMID: 39440978 Free PMC article. - Molecular evidence that GBS early neonatal sepsis results from ascending infection: comparative hybrid genomics analyses show that microorganisms in the vaginal ecosystem, amniotic fluid, chorioamniotic membranes, and neonatal blood are the same.
Pongchaikul P, Romero R, Wongsurawat T, Jenjaroenpun P, Kruasuwan W, Mongkolsuk P, Vivithanaporn P, Thaipisuttikul I, Singsaneh A, Khamphakul J, Santanirand P, Kotchompoo K, Bhuwapathanapun M, Warintaksa P, Chaemsaithong P. Pongchaikul P, et al. J Perinat Med. 2024 Oct 16;52(9):977-990. doi: 10.1515/jpm-2024-0310. Print 2024 Nov 26. J Perinat Med. 2024. PMID: 39405032
References
- Sobel JD. Is There a Protective Role for Vaginal Flora? Current infectious disease reports. 1999;1:379. - PubMed
- Witkin SS, Linhares IM, Giraldo P. Bacterial flora of the female genital tract: function and immune regulation. Best Pract Res Clin Obstet Gynaecol. 2007;21:347. - PubMed
- Yamamoto T, Zhou X, Williams CJ, Hochwalt A, Forney LJ. Bacterial populations in the vaginas of healthy adolescent women. J Pediatr Adolesc Gynecol. 2009;22:11. - PubMed
- Dunstan PK, Johnson CR. Linking richness, community variability, and invasion resistance with patch size. Ecology. 2006;87:2842. - PubMed
Publication types
MeSH terms
Grants and funding
- U01-AI70921/AI/NIAID NIH HHS/United States
- UH2 AI083264/AI/NIAID NIH HHS/United States
- UH2-AI083264/AI/NIAID NIH HHS/United States
- K01-AI080974/AI/NIAID NIH HHS/United States
- U01 AI070921/AI/NIAID NIH HHS/United States
- K01 AI080974/AI/NIAID NIH HHS/United States
- R03-AI061131/AI/NIAID NIH HHS/United States
- R03 AI061131/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources