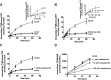Augmentation of reduced folate carrier-mediated folate/antifolate transport through an antiport mechanism with 5-aminoimidazole-4-carboxamide riboside monophosphate - PubMed (original) (raw)
Augmentation of reduced folate carrier-mediated folate/antifolate transport through an antiport mechanism with 5-aminoimidazole-4-carboxamide riboside monophosphate
Michele Visentin et al. Mol Pharmacol. 2012 Aug.
Abstract
5-Aminoimidazole-4-carboxamide riboside (AICAR), an agent with diverse pharmacological properties, augments transport of folates and antifolates. This report further characterizes this phenomenon and defines the mechanism by which it occurs. Exposure of HeLa cells to AICAR resulted in augmentation of methotrexate, 5-formyltetrahydrofolate, and 5-methyltetrahydrofolate initial rates and net uptake in cells that express the reduced folate carrier (RFC). This did not occur in cells that express only the proton-coupled folate transporter and accumulated folates by this mechanism. Transport stimulation correlated with the accumulation of 5-aminoimidazole-4-carboxamide ribotide monophosphate (ZMP), the monophosphate derivative of AICAR, within cells as established by liquid chromatography. When ZMP formation was blocked with 5-iodotubercidin, an inhibitor of adenosine kinase, folate transport stimulation by AICAR was absent. When cells first accumulated ZMP and were then exposed to 5-iodotubercidin or AICAR-free buffer, the ZMP level markedly decreased and folate transport stimulation was abolished. Extracellular ZMP inhibited RFC-mediated folate influx, and the presence of intracellular ZMP correlated with inhibition of folate efflux. The data indicate that intracellular ZMP trans-stimulates folate influx and inhibits folate efflux, which, together, produce a marked augmentation in the net cellular folate level. This interaction among ZMP, folates, and RFC, a folate/organic phosphate antiporter, is consistent with a classic exchange reaction. The transmembrane gradient for one transport substrate (ZMP) drives the uphill transport of another (folate) via a carrier used by both substrates, a phenomenon intrinsic to the energetics of RFC-mediated folate transport.
Figures
Fig. 1.
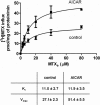
Impact of 1 mM AICAR on folate influx and net uptake in HeLa cells. Transport of 0.5 μM [3H]MTX (left), [3H]5-formyl-THF (center), and [3H]5-methyl-THF (right) in the presence and absence of 1 mM AICAR. AICAR was added 10 min before addition of folates and was present during the transport measurements. Top, initial rates of folate uptake (influx). Bottom, the time course of the net uptake of folates. The horizontal interrupted line in bottom left represents MTX tightly bound to dihydrofolate reductase. The different between bound and total intracellular MTX is the component free within the intracellular water (see Materials and Methods). The data are from an experiment representative of three independent experiments.
Fig. 2.
Roles of RFC and PCFT in AICAR potentiation of net folate transport. Top, net uptake of 0.5 μM [3H]MTX (left) and [3H]5-formyl-THF (right) in the presence and absence of 1 mM AICAR in cells that express only RFC (HeLa-RFC6). Bottom, net uptake of 0.5 μM [3H]MTX (left) and [3H]5-formyl-THF (right) in the presence and absence of 1 mM AICAR in cells that express only PCFT (HeLa-PCFT). AICAR was added 10 min before addition of folates and was present during the transport measurements. The data are representative of three independent experiments.
Fig. 3.
Impact of AICAR on RFC influx kinetics. HeLa cells were incubated in the presence or absence of 1 mM AICAR for 10 min after which influx of [3H]MTX was assessed in the presence of this agent. The line is best fit to the Michaelis-Menten equation (V = _V_max[_S_]/(_K_t+[_S_]). _K_t is in micromoles per liter and represents the extracellular concentration (MTXe) at which MTX influx is half of maximum; _V_max is in picomoles per milligram of protein per minute. Results are the mean ± S.E.M. from three independent experiments.
Fig. 4.
The impact of 5-iodotubercidin and the reversibility of AICAR stimulation of RFC-mediated transport. A, cells were preincubated for 10 min with 1 mM AICAR and then were incubated for 30 min in AICAR-free buffer after which uptake of 0.5 μM [3H]5-formyl-THF was assessed. Results are the mean ± S.E.M. from three independent experiments. The inset amplifies the initial uptake over 5 min. B, cells were preincubated for 10 min with 1 mM AICAR and then were exposed to AICAR-free buffer at time 0, and uptake of [3H]5-formyl-THF was assessed. The inset amplifies uptake over the first 15 min. Results are the mean ± S.E.M. from three independent experiments. C, time course of uptake of 0.5 μM [3H]5-formyl-THF in HeLa cells preincubated for 10 min with 1 mM AICAR in the presence and absence (control) of 1 μM 5-iodotubercidin. Data are the mean ± S.E.M. from three independent experiments. D, initial rate of 1 mM [3H]AICAR uptake in HeLa cells as a function of 5-iodotubercidin concentration. Data are the mean ± S.E.M. from three independent experiments.
Fig. 5.
HPLC analysis of intracellular constituents after incubation of cells with [3H]AICAR. A, HeLa cells were incubated for 1 h with 1 mM [3H]AICAR. B, cells were treated as in A but in the presence of 1 μM 5-iodotubercidin. C, cells were incubated for 1 h with 1 mM [3H]AICAR followed by a 30-min incubation with 1 μM 5-iodotubercidin. D, cells were incubated with 1 mM [3H]AICAR for 1 h and then were incubated for 30 min more in AICAR-free buffer. The chromatograms reflect a representative experiment. The legend quantifies the results from three independent experiments, showing the average value of each intracellular constituent ± S.E.M.
Fig. 6.
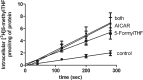
Impact of AICAR on trans-stimulation mediated by 5-formyl-THF. HeLa cells were loaded for 20 min with 1 mM AICAR, 100 μM 5-formyl-THF, or both. The cells were then washed in 0°C HBS after which influx of 0.5 μM [3H]5-methyl-THF was assessed. Data are the mean ± S.E.M. from four independent experiments.
Fig. 7.
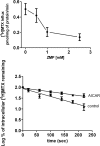
Inhibitory effect of ZMP on [3H]MTX influx and efflux. Top panel, influx of 0.5 μM [3H]MTX was assessed in HeLa cells in the absence or presence of nonlabeled ZMP at the indicated concentrations. Bottom panel, impact of AICAR on [3H]MTX efflux. HeLa cells were incubated for 30 min with 0.5 μM [3H]MTX in the presence or absence of 1 mM AICAR after which 100 μM 5-formyl-THF was added to block [3H]MTX influx, and the decline of intracellular [3H]MTX was monitored. Data are the mean ± S.E.M. from six independent experiments.
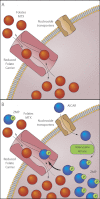
Fig. 8.
Model for a ZMP-folate antiport mechanism mediated by RFC. A, under physiological conditions intracellular ZMP levels are negligible. RFC mediates the bidirectional fluxes of folates across the cell membrane. B, after entering the cells via nucleoside transporters, AICAR is rapidly converted to ZMP by adenosine kinase. Intracellular ZMP competes with intracellular folates at the inner cell membrane for export via RFC, which results in inhibition of folate efflux and trans-stimulation of folate influx, leading to augmentation of the net level of folate accumulation within the cells.
Similar articles
- 5-aminoimidazole-4-carboxamide riboside enhances effect of ionizing radiation in PC3 prostate cancer cells.
Isebaert SF, Swinnen JV, McBride WH, Begg AC, Haustermans KM. Isebaert SF, et al. Int J Radiat Oncol Biol Phys. 2011 Dec 1;81(5):1515-23. doi: 10.1016/j.ijrobp.2011.06.1964. Epub 2011 Sep 21. Int J Radiat Oncol Biol Phys. 2011. PMID: 21944462 - Methotrexate reduces HbA1c concentration but does not produce chronic accumulation of ZMP in patients with rheumatoid or psoriatic arthritis.
Perdan-Pirkmajer K, Pirkmajer S, Thevis M, Thomas A, Praprotnik S, Hočevar A, Rotar Ž, Gašperšič N, Sodin-Šemrl S, Žibert J, Omersel J, Chibalin AV, Tomšič M, Ambrožič A. Perdan-Pirkmajer K, et al. Scand J Rheumatol. 2016 Oct;45(5):347-55. doi: 10.3109/03009742.2015.1105290. Epub 2016 Jan 4. Scand J Rheumatol. 2016. PMID: 26726793 - Recent advances in the understanding of the mechanism of membrane transport of folates and antifolates.
Sierra EE, Goldman ID. Sierra EE, et al. Semin Oncol. 1999 Apr;26(2 Suppl 6):11-23. Semin Oncol. 1999. PMID: 10598550 Review. - Human reduced folate carrier: translation of basic biology to cancer etiology and therapy.
Matherly LH, Hou Z, Deng Y. Matherly LH, et al. Cancer Metastasis Rev. 2007 Mar;26(1):111-28. doi: 10.1007/s10555-007-9046-2. Cancer Metastasis Rev. 2007. PMID: 17334909 Review.
Cited by
- Toward a better understanding of folate metabolism in health and disease.
Zheng Y, Cantley LC. Zheng Y, et al. J Exp Med. 2019 Feb 4;216(2):253-266. doi: 10.1084/jem.20181965. Epub 2018 Dec 26. J Exp Med. 2019. PMID: 30587505 Free PMC article. Review. - The membrane transport and polyglutamation of pralatrexate: a new-generation dihydrofolate reductase inhibitor.
Visentin M, Unal ES, Zhao R, Goldman ID. Visentin M, et al. Cancer Chemother Pharmacol. 2013 Sep;72(3):597-606. doi: 10.1007/s00280-013-2231-9. Epub 2013 Jul 24. Cancer Chemother Pharmacol. 2013. PMID: 23881211 Free PMC article. - Methotrexate and 5-aminoimidazole-4-carboxamide riboside exert synergistic anticancer action against human breast cancer and hepatocellular carcinoma.
Cheng XL, Zhou TY, Li B, Li MY, Li L, Li ZQ, Lu W. Cheng XL, et al. Acta Pharmacol Sin. 2013 Jul;34(7):951-9. doi: 10.1038/aps.2013.16. Epub 2013 Apr 22. Acta Pharmacol Sin. 2013. PMID: 23603981 Free PMC article. - SLC19A1 transports immunoreactive cyclic dinucleotides.
Luteijn RD, Zaver SA, Gowen BG, Wyman SK, Garelis NE, Onia L, McWhirter SM, Katibah GE, Corn JE, Woodward JJ, Raulet DH. Luteijn RD, et al. Nature. 2019 Sep;573(7774):434-438. doi: 10.1038/s41586-019-1553-0. Epub 2019 Sep 11. Nature. 2019. PMID: 31511694 Free PMC article. - Molecular mechanism of substrate recognition by folate transporter SLC19A1.
Dang Y, Zhou D, Du X, Zhao H, Lee CH, Yang J, Wang Y, Qin C, Guo Z, Zhang Z. Dang Y, et al. Cell Discov. 2022 Dec 28;8(1):141. doi: 10.1038/s41421-022-00508-w. Cell Discov. 2022. PMID: 36575193 Free PMC article.
References
- Assaraf YG. (2006) The role of multidrug resistance efflux transporters in antifolate resistance and folate homeostasis. Drug Resist Updat 9:227–246 - PubMed
- Beckers A, Organe S, Timmermans L, Vanderhoydonc F, Deboel L, Derua R, Waelkens E, Brusselmans K, Verhoeven G, Swinnen JV. (2006) Methotrexate enhances the antianabolic and antiproliferative effects of 5-aminoimidazole-4-carboxamide riboside. Mol Cancer Ther 5:2211–2217 - PubMed
- Corradetti MN, Guan KL. (2006) Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene 25:6347–6360 - PubMed
- Davies SP, Helps NR, Cohen PT, Hardie DG. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett 377:421–425 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases