Effects of temperature on emergence and seasonality of West Nile virus in California - PubMed (original) (raw)
Comparative Study
Effects of temperature on emergence and seasonality of West Nile virus in California
David M Hartley et al. Am J Trop Med Hyg. 2012 May.
Abstract
Temperature has played a critical role in the spatiotemporal dynamics of West Nile virus transmission throughout California from its introduction in 2003 through establishment by 2009. We compared two novel mechanistic measures of transmission risk, the temperature-dependent ratio of virus extrinsic incubation period to the mosquito gonotrophic period (BT), and the fundamental reproductive ratio (R(0)) based on a mathematical model, to analyze spatiotemporal patterns of receptivity to viral amplification. Maps of BT and R(0) were created at 20-km scale and compared throughout California to seroconversions in sentinel chicken flocks at half-month intervals. Overall, estimates of BT and R(0) agreed with intensity of transmission measured by the frequency of sentinel chicken seroconversions. Mechanistic measures such as these are important for understanding how temperature affects the spatiotemporal dynamics of West Nile virus transmission and for delineating risk estimates useful to inform vector control agency intervention decisions and communicate outbreak potential.
Figures
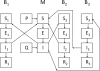
Figure 1.
Schematic of the SEIR model constructed for West Nile virus circulation in California. Birds (B) are categorized as highly competent (1), moderately competent (2), or incompetent (3), and adult mosquitoes (M) may emerge from uninfected (P) or vertically infected (Q) eggs. See the text for a complete explanation.
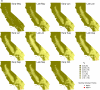
Figure 2.
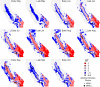
Calculated fundamental reproductive ratio (R0) estimates during the period of WestNile virus (WNV) introduction and spread in California, 2003 (A) and 2004 (B). Green colors indicate R0 estimates below or above the threshold value of 1, with less intense colors indicating values greater than 1 (see scale). For comparison of R0 values with observed transmission patterns, sentinel chicken flocks are indicated by circles, with red or white circles representing flocks with or without serological evidence of WNV transmission during each half-month, respectively.
Figure 2.
Calculated fundamental reproductive ratio (R0) estimates during the period of WestNile virus (WNV) introduction and spread in California, 2003 (A) and 2004 (B). Green colors indicate R0 estimates below or above the threshold value of 1, with less intense colors indicating values greater than 1 (see scale). For comparison of R0 values with observed transmission patterns, sentinel chicken flocks are indicated by circles, with red or white circles representing flocks with or without serological evidence of WNV transmission during each half-month, respectively.
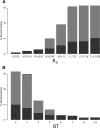
Figure 3.
Relationship between seroconversions in sentinel chickens and calculated values for fundamental reproductive ratio (R0) (A) and the temperature-dependent ratio of virus extrinsic incubation period to the mosquito gonotrophic period (BT) (B) during May–October 2003–2009. Graph shows means (height of gray bars) and 90th percentiles (total height of gray plus blue bars; if the blue bar is not visible, mean was > 90th percentile) for the percentage of chickens that seroconverted to West Nile virus in each agency over the range of R0 values calculated in this study.
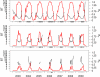
Figure 4.
R0 as a function of time (plotted in red) for representative mosquito control districts along the cool northern coast of California (Marin-Sonoma; bottom panel), in the warm Central Valley (Sacramento-Yolo; middle panel), and in the hot southeastern deserts (Coachella Valley; top panel). BT, the ratio of extrinsic incubation period to the gonotrophic period as a function of time, is plotted in black. Values of BT are shown only for periods when West Nile virus (WNV) amplification is theoretically possible (i.e., when temperatures were above the WNV replication threshold of 14.3°C). For comparison, sentinel chicken flocks are indicated along the x-axis by circles, with black and gray circles representing flocks with or without serologic evidence of WNV transmission, respectively. R0 = fundamental reproductive ratio.
Figure 5.
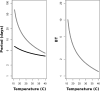
A, Red = extrinsic incubation period (EIP); black = gonotrophic period (GP). B, Ratio of EIP to GP (ratio of extrinsic incubation period to the gonotrophic period as a function of time). In both panels, the red vertical line denotes 14.3°C, the replication threshold of West Nile virus.
Figure 6.
BT, the ratio of extrinsic incubation period to gonotrophic period for West Nile virus (WNV) based on estimated mean mosquito exposure temperatures during 2004 as WNV spread northward across California. Hatched areas are below the estimated threshold for WNV replication in mosquitoes. Serologic evidence of WNV transmission to sentinel chickens is shown for comparison of extrinsic incubation periods with observed transmission patterns, with closed and open circles representing flocks with or without serologic evidence of WNV transmission during the half-month, respectively. N/A = not applicable.
Figure 7.
Proportion of mosquitoes surviving the minimum number of gonotrophic cycles required for West Nile virus transmission (temperature-dependent ratio of virus extrinsic incubation period to the mosquito gonotrophic period [BT]) after infection for the estimated mosquito exposure temperatures in this study. Because survival decreases with increasing temperature (daily survival = 0.943 − 0.0092 × temperature), for each value of BT, the greatest proportionate survival occurs at the lower end of the temperature range.
Similar articles
- [Usutu virus in Austria reminds one of the West Nile virus in the USA].
Hassler D, Pfeffer M, Weissenböck H, Nowotny N. Hassler D, et al. Dtsch Med Wochenschr. 2004 Oct 29;129(44):2339-40. Dtsch Med Wochenschr. 2004. PMID: 15822217 Review. German. No abstract available. - Overwintering of West Nile virus in Southern California.
Reisen WK, Fang Y, Lothrop HD, Martinez VM, Wilson J, Oconnor P, Carney R, Cahoon-Young B, Shafii M, Brault AC. Reisen WK, et al. J Med Entomol. 2006 Mar;43(2):344-55. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. J Med Entomol. 2006. PMID: 16619621 - Surveillance for West Nile virus in British birds (2001 to 2006).
Phipps LP, Duff JP, Holmes JP, Gough RE, McCracken F, McElhinney LM, Johnson N, Hughes L, Chantrey J, Pennycott T, Murray KO, Brown IH, Fooks AR. Phipps LP, et al. Vet Rec. 2008 Mar 29;162(13):413-5. doi: 10.1136/vr.162.13.413. Vet Rec. 2008. PMID: 18375986 No abstract available. - Modelling the dynamics of West Nile Virus.
Cruz-Pacheco G, Esteva L, Montaño-Hirose JA, Vargas C. Cruz-Pacheco G, et al. Bull Math Biol. 2005 Nov;67(6):1157-72. doi: 10.1016/j.bulm.2004.11.008. Bull Math Biol. 2005. PMID: 16125762 - West Nile virus infections in Greece: an update.
Papa A. Papa A. Expert Rev Anti Infect Ther. 2012 Jul;10(7):743-50. doi: 10.1586/eri.12.59. Expert Rev Anti Infect Ther. 2012. PMID: 22943398 Review.
Cited by
- Identification of Climatic Factors Affecting the Epidemiology of Human West Nile Virus Infections in Northern Greece.
Stilianakis NI, Syrris V, Petroliagkis T, Pärt P, Gewehr S, Kalaitzopoulou S, Mourelatos S, Baka A, Pervanidou D, Vontas J, Hadjichristodoulou C. Stilianakis NI, et al. PLoS One. 2016 Sep 15;11(9):e0161510. doi: 10.1371/journal.pone.0161510. eCollection 2016. PLoS One. 2016. PMID: 27631082 Free PMC article. - Effect of Trapping Methods, Weather, and Landscape on Estimates of the Culex Vector Mosquito Abundance.
Karki S, Hamer GL, Anderson TK, Goldberg TL, Kitron UD, Krebs BL, Walker ED, Ruiz MO. Karki S, et al. Environ Health Insights. 2016 Jun 22;10:93-103. doi: 10.4137/EHI.S33384. eCollection 2016. Environ Health Insights. 2016. PMID: 27375359 Free PMC article. - An epidemiological model for mosquito host selection and temperature-dependent transmission of West Nile virus.
Fasano A, Riccetti N, Angelou A, Gomez-Ramirez J, Ferraccioli F, Kioutsioukis I, Stilianakis NI. Fasano A, et al. Sci Rep. 2022 Nov 19;12(1):19946. doi: 10.1038/s41598-022-24527-5. Sci Rep. 2022. PMID: 36402904 Free PMC article. - Data-driven modeling to assess receptivity for Rift Valley Fever virus.
Barker CM, Niu T, Reisen WK, Hartley DM. Barker CM, et al. PLoS Negl Trop Dis. 2013 Nov 14;7(11):e2515. doi: 10.1371/journal.pntd.0002515. eCollection 2013 Nov. PLoS Negl Trop Dis. 2013. PMID: 24244769 Free PMC article. - Comparative fitness of West Nile virus isolated during California epidemics.
Worwa G, Hutton AA, Brault AC, Reisen WK. Worwa G, et al. PLoS Negl Trop Dis. 2019 Feb 4;13(2):e0007135. doi: 10.1371/journal.pntd.0007135. eCollection 2019 Feb. PLoS Negl Trop Dis. 2019. PMID: 30716113 Free PMC article.
References
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, Mackenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. - PubMed
- Hom A, Marcus L, Kramer VL, Cahoon BE, Glaser C, Cossen C, Baylis E, Jean C, Tu EH, Eldridge BF, Carney R, Padgett K, Sun B, Reisen WK, Woods L, Husted S. Surveillance for mosquito-borne encephalitis virus activity and human disease, including West Nile virus in California, 2004. Proc Mosq Vector Control Assoc Calif. 2005;73:66–77.
- California Department of Public Health California West Nile Virus. 2010. http://westnile.ca.gov Available at. Accessed March 24, 2011.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical