Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function - PubMed (original) (raw)
Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function
Swapnil K Sonkusare et al. Science. 2012.
Abstract
Major features of the transcellular signaling mechanism responsible for endothelium-dependent regulation of vascular smooth muscle tone are unresolved. We identified local calcium (Ca(2+)) signals ("sparklets") in the vascular endothelium of resistance arteries that represent Ca(2+) influx through single TRPV4 cation channels. Gating of individual TRPV4 channels within a four-channel cluster was cooperative, with activation of as few as three channels per cell causing maximal dilation through activation of endothelial cell intermediate (IK)- and small (SK)-conductance, Ca(2+)-sensitive potassium (K(+)) channels. Endothelial-dependent muscarinic receptor signaling also acted largely through TRPV4 sparklet-mediated stimulation of IK and SK channels to promote vasodilation. These results support the concept that Ca(2+) influx through single TRPV4 channels is leveraged by the amplifier effect of cooperative channel gating and the high Ca(2+) sensitivity of IK and SK channels to cause vasodilation.
Conflict of interest statement
The authors have no conflicts of interest.
Figures
Fig. 1
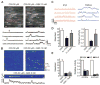
GSK-induced Ca2+ signals represent Ca2+ influx through plasmalemmal TRPV4 channels. (A) Ca2+ imaging of an en face preparation of third-order mesenteric arteries from a GCaMP2-expressing mouse, showing changes in the activity of local, IP3R-independent Ca2+ signals recorded over time (traces, below) from regions of interest (1.7 μm2), denoted by boxes in images (above). An EC in the field is outlined by red dashes (above, left). Scale bar: 10 μm. Bottom left: Localized events (arrows) detected in the absence of IP3R-mediated signaling; bottom right: localized events after addition of GSK (10 nM). (B) Top panels: Pseudocolor overlay images showing all IP3R-independent Ca2+ signaling events detected in the absence or presence of GSK (10 nM) in a single field over a 94-s interval. Scale bar: 10 μm. Bottom panel and trace: Pseudo–line-scan image and associated trace recorded from a single site over the same interval. Scale bar: 2 s. Calibration bar at right indicates intensity of signals (F/_F_0; fluorescence F divided by baseline fluorescence _F_0). (C) Representative traces illustrating differences in the kinetic properties of IP3R-mediated Ca2+ pulsars (left) and TRPV4-mediated Ca2+ events (right) from the same field of view in the presence of 10 nM GSK (without CPA). (D) Increased numbers of sites per 2 min per field and activity per field (right; n = 4 to 6 arteries) in the presence of GSK (10 nM), 4α-PDD (5 μM), or 11,12-EET (1 μM). (E) Inhibition of GSK-induced increases in activity per site and activity per field by HC (1 μM; n = 5) and RuR (5 μM; n = 3). Error bars (C and D), SEMs.
Fig. 2
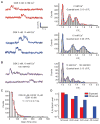
TRPV4 Ca2+ sparklets as unitary events that exhibit cooperative activation. (A) A fit of multiple Gaussians to all-points histograms showing evenly spaced Δ_F_/_F_0 levels of 0.19 at 2 mM extracellular Ca2+ and 0.29 at 10 mM Ca2+. (B) Effect of decreasing Ca2+ electrochemical gradient by membrane depolarization with 100 mM K+ on Ca2+ signals. (C) Exponential distribution of the durations of GSK (10 nM)–induced sparklets with Fluo-4 and 10 μM EGTA-AM. Sampling rate, 50 to 60 images/s. τ, time constant. (D) Cooperative channel gating demonstrated by deviation of the frequencies of second, third, and fourth levels (measured as open probabilities, _P_O) from a binomial distribution predictive of independent events. Same conditions as in (C).
Fig. 3
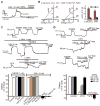
Activation of IK and SK channels and induction of EDHF-dependent vasodilation by Ca2+ influx through TRPV4 channels. (A) Inhibition of GSK (10 nM)–induced outward current by 300 nM ChTx (n = 5 cells) or 300 nM apamin (n = 5 cells). (B) Densities of IK and SK channel currents in cells treated with GSK (10 nM) or in cells dialyzed with 3 μM Ca2+ (n = 8 cells). Conv. W.C., conventional whole-cell configuration; Perf. Patch, perforated-patch configuration. (C) Top trace: GSK (3 nM)–induced vasodilation of pressurized (80 mmHg) mesenteric arteries; middle trace: effects of 100 μM L-NNA + 10 μM indomethacin (Indo; n = 5), or 1 μM paxilline (n = 3) on GSK-induced dilations; bottom trace: effects of L-NNA and 30 μM CPA (n = 5) on GSK (10 nM)–induced dilations; bar graph (left): absence of effects of the TRPV4 antagonists HC (1 μM; n = 4) and RuR (5 μM; n = 4), and TRPV4 knockout (n = 3) on dilations induced by the IK and SK agonist NS309 (1 μM); bar graph (right): quantification of effects of CPA + L-NNA (n = 5), L-NNA + Indo (n = 5), paxilline (n = 3), endothelium removal (n = 5), RuR (n = 5), HC (n = 5), and TRPV4 knockout (n = 4) on GSK (10 nM)–induced dilations. (D) Vasodilation in response to GSK (3, 10, 30 nM) in the presence or absence of 200 nM ChTx (n = 7), or ChTx + 300 nM apamin (Apa). Effects on dilation [bar graphs in (C) and (D)] determined relative to initial tone [26 ± 1% (SEM), n = 10], defined as the percentage decrease in arterial diameter to pressure (80 mmHg) relative to the diameter in external Ca2+-free solution. Error bars (B to D), SEMs.
Fig. 4
Effects of muscarinic receptor activation on global Ca2+ and TRPV4 sparklets. (A) Global Ca2+, shown as fractional fluorescence from outlined whole cells treated with CCh (10 μM) with and without CPA (30 μM). (B) GSK (3 nM)–induced sparklets in the presence or absence of 5 μM ACh (left) and 1 μM HC (right; n = 5). (C) Effects of 200 nM ChTx + 300 nM apamin (Apa; n = 5), 100 μM L-NNA (n = 6), L-NNA + HC (n = 5), and ChTx + Apa + L-NNA (n = 4) on dilations in response to the muscarinic agonist CCh (1 μM). Error bars (B and C), SEMs. Except where indicated by a bracket (Student’s t test), _P_-values are for comparison to control (one-way analysis of variance).
Comment in
- Cell biology. Superresolution subspace signaling.
Lederer WJ, Hagen BM, Zhao G. Lederer WJ, et al. Science. 2012 May 4;336(6081):546-7. doi: 10.1126/science.1222540. Science. 2012. PMID: 22556238 Free PMC article. No abstract available.
Similar articles
- Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels.
Naik JS, Osmond JM, Walker BR, Kanagy NL. Naik JS, et al. Am J Physiol Heart Circ Physiol. 2016 Dec 1;311(6):H1437-H1444. doi: 10.1152/ajpheart.00465.2016. Epub 2016 Oct 7. Am J Physiol Heart Circ Physiol. 2016. PMID: 27765747 Free PMC article. - Physiological levels of fluid shear stress modulate vascular function through TRPV4 sparklets.
Geng L, Zhang C, He C, Zhang K, Kan H, Mao A, Ma X. Geng L, et al. Acta Biochim Biophys Sin (Shanghai). 2022 Sep 25;54(9):1268-1277. doi: 10.3724/abbs.2022118. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36082933 Free PMC article. - Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators.
Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Sonkusare SK, et al. J Physiol. 2016 Jun 15;594(12):3271-85. doi: 10.1113/JP271652. Epub 2016 Mar 4. J Physiol. 2016. PMID: 26840527 Free PMC article. - Endothelium-dependent cerebral artery dilation mediated by transient receptor potential and Ca2+-activated K+ channels.
Earley S. Earley S. J Cardiovasc Pharmacol. 2011 Feb;57(2):148-53. doi: 10.1097/FJC.0b013e3181f580d9. J Cardiovasc Pharmacol. 2011. PMID: 20729757 Review. - TRPV4 and the regulation of vascular tone.
Filosa JA, Yao X, Rath G. Filosa JA, et al. J Cardiovasc Pharmacol. 2013 Feb;61(2):113-9. doi: 10.1097/FJC.0b013e318279ba42. J Cardiovasc Pharmacol. 2013. PMID: 23107877 Free PMC article. Review.
Cited by
- Impairment of IKCa channels contributes to uteroplacental endothelial dysfunction in rat diabetic pregnancy.
Gokina NI, Bonev AD, Phillips J, Gokin AP, Veilleux K, Oppenheimer K, Goloman G. Gokina NI, et al. Am J Physiol Heart Circ Physiol. 2015 Aug 15;309(4):H592-604. doi: 10.1152/ajpheart.00901.2014. Epub 2015 Jun 19. Am J Physiol Heart Circ Physiol. 2015. PMID: 26092991 Free PMC article. - Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca²⁺-activated K⁺ channels and smooth muscle Ca²⁺ sparks.
Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Jackson-Weaver O, et al. Am J Physiol Heart Circ Physiol. 2013 Jun 1;304(11):H1446-54. doi: 10.1152/ajpheart.00506.2012. Epub 2013 Mar 22. Am J Physiol Heart Circ Physiol. 2013. PMID: 23525712 Free PMC article. - A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFα-induced increases in endothelial permeability.
Maier-Begandt D, Comstra HS, Molina SA, Krüger N, Ruddiman CA, Chen YL, Chen X, Biwer LA, Johnstone SR, Lohman AW, Good ME, DeLalio LJ, Hong K, Bacon HM, Yan Z, Sonkusare SK, Koval M, Isakson BE. Maier-Begandt D, et al. Sci Signal. 2021 Mar 2;14(672):eaba2940. doi: 10.1126/scisignal.aba2940. Sci Signal. 2021. PMID: 33653920 Free PMC article. - Role of TRP Channels in Metabolism-Related Diseases.
Wu F, Bu S, Wang H. Wu F, et al. Int J Mol Sci. 2024 Jan 5;25(2):692. doi: 10.3390/ijms25020692. Int J Mol Sci. 2024. PMID: 38255767 Free PMC article. Review. - Mechanisms of enhanced basal tone of brain parenchymal arterioles during early postischemic reperfusion: role of ET-1-induced peroxynitrite generation.
Cipolla MJ, Sweet JG, Gokina NI, White SL, Nelson MT. Cipolla MJ, et al. J Cereb Blood Flow Metab. 2013 Oct;33(10):1486-92. doi: 10.1038/jcbfm.2013.99. Epub 2013 Jun 19. J Cereb Blood Flow Metab. 2013. PMID: 23778163 Free PMC article.
References
- Saliez J, et al. Circulation. 2008;117:1065. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM086736/GM/NIGMS NIH HHS/United States
- 2-P20-RR-016435-06/RR/NCRR NIH HHS/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- P20 RR016435/RR/NCRR NIH HHS/United States
- R01 GM086736/GM/NIGMS NIH HHS/United States
- R01HL098243/HL/NHLBI NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- HL044455/HL/NHLBI NIH HHS/United States
- R01 HL098243/HL/NHLBI NIH HHS/United States
- 1P01HL095488/HL/NHLBI NIH HHS/United States
- R37DK053832/DK/NIDDK NIH HHS/United States
- R01 HL044455/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous