Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke - PubMed (original) (raw)
Review
Sex, stroke, and inflammation: the potential for estrogen-mediated immunoprotection in stroke
Rodney M Ritzel et al. Horm Behav. 2013 Feb.
Abstract
Stroke is the third leading cause of death and the primary cause of disability in the developed world. Experimental and clinical data indicate that stroke is a sexually dimorphic disease, with males demonstrating an enhanced intrinsic sensitivity to ischemic damage throughout most of their lifespan. The neuroprotective role of estrogen in the female brain is well established, however, estrogen exposure can also be deleterious, especially in older women. The mechanisms for this remain unclear. Our current understanding is based on studies examining estrogen as it relates to neuronal injury, yet cerebral ischemia also induces a robust sterile inflammatory response involving local and systemic immune cells. Despite the potent anti-inflammatory effects of estrogen, few studies have investigated the contribution of estrogen to sex differences in the inflammatory response to stroke. This review examines the potential role for estrogen-mediated immunoprotection in ischemic injury.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
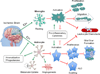
Figure 1. The potential estrogenic effects on glial cells in the ischemic brain
The anti-inflammatory effects of estrogen in the brain are primarily mediated by glial cells. Estrogen promotes a resting phenotype in microglia, as evidenced by increased ramified morphology and decreased MHCII expression. These actions serve to limit microglial activation, proliferation, and migration toward the injured site. Similar changes occur in astrocytes, ultimately leading to decreased glial scar formation. Estrogen-mediated decrease in aquaporin expression prevents osmotic imbalance and may lower risk of edema. Pro-inflammatory cytokines and chemoattractant signals normally released by activated astrocytes and microglia are suppressed in the presence of estrogen, thereby reducing extravasation of infiltrating immune cells. As well, estrogen enhances astrocytic expression of glutamate transporters which facilitates glutamate uptake, mitigating injury due to neuronal excitoxicity. In addition, astrocytes increase synthesis of endogenous steroid production after injury, and secrete growth factors like VEGF, which promote angiogenesis. Together, these actions have a profound impact on curbing neuronal injury due to ischemia by conferring protection via local immunosuppression. The green arrow represents enhancing or promoting action, and the red arrow represents inhibitory action. Abbreviations: E2 (17 beta-estradiol), MHCII (major histocompatibility complex class II), VEGF (vascular endothelial growth factor)
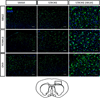
Figure 2. Microglial activation after stroke
Coronal section of mouse brain at low (10×) and high (20×) power demonstrating increased Iba staining (a marker for microglia) in the penumbral region (indicated by a box in the schematic section) in ovariectomized females both at baseline (sham) and after stroke. DAPI was utilized as a nuclear counter stain.
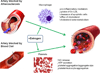
Figure 3. The atheroprotective effects of estrogen
Primary and secondary stroke prevention centers around vascular dysfunction associated with atherosclerosis and blood clotting, respectively. Macrophages are key players in atherogenesis, the development of arterial plaques. Estrogen acts to suppress pro-inflammatory cytokine production, and enhance removal of dead cells from the atherosclerotic tissue. Decreased uptake of modified lipoproteins prevents foam cell formation. Estrogen also prevents lipid buildup by increasing cholesterol ester metabolism and enhancing efflux transport of cholesterol out of the cell. Excessive blood clotting in response to vascular injury following stroke can impede reperfusion of blood flow back to ischemic tissue. Platelets are major contributors in the formation of blood clots. Estrogen has been shown to increase platelet- and endothelial derived NO levels, which plays an important role in reducing adhesion, aggregation, and recruitment. Decreased platelet-leukocyte aggregation due to estrogen exposure prevents lesion progression, plaque rupture, thrombus formation, and the risk of embolization. In sum, these changes prevent clotting and re-establish blood flow back to the ischemic site. The green arrow represents enhancing or promoting action. Abbreviations: Ox-LDL (oxidized low-density lipoprotein), NO (nitric oxide), ATP (adenosine triphosphate)
Similar articles
- The effects of estrogen in ischemic stroke.
Koellhoffer EC, McCullough LD. Koellhoffer EC, et al. Transl Stroke Res. 2013 Aug;4(4):390-401. doi: 10.1007/s12975-012-0230-5. Epub 2012 Dec 7. Transl Stroke Res. 2013. PMID: 24323337 Free PMC article. Review. - Experimental pediatric arterial ischemic stroke model reveals sex-specific estrogen signaling.
Herson PS, Bombardier CG, Parker SM, Shimizu T, Klawitter J, Klawitter J, Quillinan N, Exo JL, Goldenberg NA, Traystman RJ. Herson PS, et al. Stroke. 2013 Mar;44(3):759-63. doi: 10.1161/STROKEAHA.112.675124. Epub 2013 Jan 24. Stroke. 2013. PMID: 23349190 Free PMC article. - Elucidating sex differences in response to cerebral ischemia: immunoregulatory mechanisms and the role of microRNAs.
Kaidonis G, Rao AN, Ouyang YB, Stary CM. Kaidonis G, et al. Prog Neurobiol. 2019 May;176:73-85. doi: 10.1016/j.pneurobio.2018.08.001. Epub 2018 Aug 16. Prog Neurobiol. 2019. PMID: 30121237 Review. - Cerebral stroke injury: the role of cytokines and brain inflammation.
Siniscalchi A, Gallelli L, Malferrari G, Pirritano D, Serra R, Santangelo E, De Sarro G. Siniscalchi A, et al. J Basic Clin Physiol Pharmacol. 2014 May 1;25(2):131-7. doi: 10.1515/jbcpp-2013-0121. J Basic Clin Physiol Pharmacol. 2014. PMID: 24515999 Review. - Estrogen, neuroprotection and neurogenesis after ischemic stroke.
Shao B, Cheng Y, Jin K. Shao B, et al. Curr Drug Targets. 2012 Feb;13(2):188-98. doi: 10.2174/138945012799201702. Curr Drug Targets. 2012. PMID: 22204318 Review.
Cited by
- J-shaped associations of pan-immune-inflammation value and systemic inflammation response index with stroke among American adults with hypertension: evidence from NHANES 1999-2020.
Chen J, Luo C, Tan D, Li Y. Chen J, et al. Front Neurol. 2024 Jul 31;15:1417863. doi: 10.3389/fneur.2024.1417863. eCollection 2024. Front Neurol. 2024. PMID: 39144717 Free PMC article. - Gut-Brain Axis: Focus on Sex Differences in Neuroinflammation.
Caldarelli M, Rio P, Marrone A, Ocarino F, Chiantore M, Candelli M, Gasbarrini A, Gambassi G, Cianci R. Caldarelli M, et al. Int J Mol Sci. 2024 May 15;25(10):5377. doi: 10.3390/ijms25105377. Int J Mol Sci. 2024. PMID: 38791415 Free PMC article. Review. - Spatiotemporal Cofilin Signaling, Microglial Activation, Neuroinflammation, and Cognitive Impairment Following Hemorrhagic Brain Injury.
Almarghalani DA, Sha X, Mrak RE, Shah ZA. Almarghalani DA, et al. Cells. 2023 Apr 13;12(8):1153. doi: 10.3390/cells12081153. Cells. 2023. PMID: 37190062 Free PMC article. - Immune regulation based on sex differences in ischemic stroke pathology.
Niu P, Li L, Zhang Y, Su Z, Wang B, Liu H, Zhang S, Qiu S, Li Y. Niu P, et al. Front Immunol. 2023 Jan 30;14:1087815. doi: 10.3389/fimmu.2023.1087815. eCollection 2023. Front Immunol. 2023. PMID: 36793730 Free PMC article. Review. - Exogenous inter-α inhibitor proteins prevent cell death and improve ischemic stroke outcomes in mice.
McCullough LD, Roy-O'Reilly M, Lai YJ, Patrizz A, Xu Y, Lee J, Holmes A, Kraushaar DC, Chauhan A, Sansing LH, Stonestreet BS, Zhu L, Kofler J, Lim YP, Venna VR. McCullough LD, et al. J Clin Invest. 2021 Sep 1;131(17):e144898. doi: 10.1172/JCI144898. J Clin Invest. 2021. PMID: 34580244 Free PMC article.
References
- Adamski J, Ma Z, Nozell S, Benveniste EN. 17beta-Estradiol inhibits class II major histocompatibility complex (MHC) expression: influence on histone modifications and cbp recruitment to the class II MHC promoter. Mol Endocrinol. 2004;18:1963–1974. - PubMed
- Akopov SE, Simonian NA, Grigorian GS. Dynamics of polymorphonuclear leukocyte accumulation in acute cerebral infarction and their correlation with brain tissue damage. Stroke; a journal of cerebral circulation. 1996;27:1739–1743. - PubMed
- Al-Bader MD, Malatiali SA, Redzic ZB. Expression of estrogen receptor alpha and beta in rat astrocytes in primary culture: effects of hypoxia and glucose deprivation. Physiological research/Academia Scientiarum Bohemoslovaca. 2011 - PubMed
- Altinoz MA, Korkmaz R. NF-kappaB, macrophage migration inhibitory factor and cyclooxygenase-inhibitions as likely mechanisms behind the acetaminophen- and NSAID-prevention of the ovarian cancer. Neoplasma. 2004;51:239–247. - PubMed
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O'Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA : the journal of the American Medical Association. 2004;291:1701–1712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 NS083244/NS/NINDS NIH HHS/United States
- R01 NS055215/NS/NINDS NIH HHS/United States
- R21 NS066406/NS/NINDS NIH HHS/United States
- R21 NS076293/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical